Pharmaceutical products quality assessments – future!
Pharmaceutical products quality assessments – future!
What should one expect, after FDA completely destroyed, and rightly so, the credibility and usefulness as well as need for bioequivalence assessment (aka clinical trials) by removing its requirement from ANDA approvals, at least for hydroxychloroquine (HCQ) and chloroquine (CQ) products to start with [1, 2]? It is to be noted that bioequivalence assessments have been shown to be scientifically invalid and irrelevant to establish the quality of the products based on their drug release assessment [3]. Not only such studies put large burden, financial and personnel, on the industry as well regulatory authorities for the development, manufacturing and approval of products but also expose healthy human subjects, in particular young adults, to potent chemicals (a serious unethical practice).
Products can easily and accurately be assessed using drug dissolution testing. However, to implement its (drug dissolution testing) valid use authorities, including FDA and USP, need to implement appropriate and scientifically valid dissolution testers and methods. Until then authorities’ claims regarding monitoring and establishing products quality will remain false and invalid.
COVID-19 pandemic exposed the burdensome and unnecessary regulatory practices and requirements. It also provides an opportunity to simplify the product development and manufacturing. Please consider the under-review Citizen Petition for removing the use of non-validated/non-qualified, hence non-GMP, USP drug dissolution testers/tests from regulatory requirements [4] and replace them with appropriate and scientifically valid tests and testers [5].
What is wrong with the following scenario?
What is wrong with the following scenario?
We, the authorities and experts, are extremely sorry to let you know that we made a colossal mistake in declaring the Corona virus (COVID-19) pandemic. It is one of such rare situations where we found out at an extremely high cost (human and financial) that our scientific understanding of the subject was flawed and failed us. We honestly carried away with our projections and fear of huge number of anticipated deaths by the potential spread of this variant flu which clearly did not come out to be correct. We sincerely regret this unfortunate situation and ask for your forgiveness. Please accept our apologies. We hope that public will forgive us and will start its usual daily life. In addition, we hope that public would avoid the use and/or development of new medicines based on our falsely classified disease and pandemic hence reducing any further damages which may occur.
Once again, we sincerely apologise and hope that we will be forgiven.
Clinical trials – credibility issue?
Clinical trials – credibility issue?
In general clinical trials are important and necessary. It is like in any other area that one has to show that the “things” (in this case, medicines/treatments) work as expected – clinical trials serve such purpose.
However, in medicines area, underlying scientific concepts and practices are extremely poor hence “clinical trials” practices face credibility issue. For example, developing products (tablet/capsule) clinical trials (bioequivalence test – regulatory requirement) are conducted which indeed lack clinical relevance and usefulness. Therefore, it could be argued that such tests indeed expose subjects, often healthy human volunteers, needlessly to potent chemicals in the name of medicines development. (link)
Similarly, relating to Corona virus pandemic, there appears to be rush towards development of medicines/vaccines. It may be argued that as the underlying analytical science is not well-established to monitor virus and/or its “disease” it would be very difficult to conduct appropriate and validated “clinical trials” (link)
In short, running clinical trials is a good idea, however, conducting appropriate and useful clinical trials remains challenging that is where the confusion is.
Coronavirus pandemic: Public/patients deserve better!
Coronavirus pandemic: Public/patients deserve better!
The unfortunate situation created by this Coronavirus pandemic is providing a serious opportunity for reassessing the current regulatory approaches in pharmaceutical products development as well as their manufacturing so that in future such irrelevant discussion can be avoided and patients can have access to modern and multiple options to treat ailments. Hopefully in the future patients will be treated with well-established products rather than products developed on the fly or with the use of disposable gowns, masks, washing hands and/or staying home policy which certainly are not the treatments – patients expect and deserve something better from us as scientists, physicians and regulators. Follow the link for complete article (link)
Bye Bye – Bioequivalence testing? Long live drug dissolution testing! —– #2 (gift from heaven)
Bye Bye – Bioequivalence testing? Long live drug dissolution testing! —– #2 (gift from heaven)
Yesterday, I posted my view on the recent FDA guidance documents for chloroquine and hydroxychloroquine (link). I do not think people realize the long term impact of this development where BE studies have been replaced/substituted with drug dissolution testing. Let me explain:
- Saying that guidances are product specific is not correct, because chloroquine and hydroxychloroquine are drugs not products. Products are tablets, or capsules, with often unknown and proprietary composition of a drug, excipients and manufacturing attributes (i.e. formulation and manufacturing attributes). Hence, guidances cannot be product specific as assumed or suggested.
- A drug dissolution test is conducted for products not drugs. As product attributes are mostly unknown and propriety, as noted above, hence a dissolution test (or guidance) cannot be product specific but has to be independent “standard or universal”.
- Furthermore, it is to be noted that in principle product specific guidance concept is an invalid concept. Drug dissolution testing is a scale used to measure dissolution characteristics of a product. By definition it (scale) has to be independent to the tested items. Point being that the guidance documents cannot be restricted one or two drug products. These have to be applicable to ALL highly soluble drug products. It would not be possible for authorities, at least scientifically, to defend restricting to only one or two products. This decision could easily be challenged and won.
- In addition, such a decision cannot be one time decision, as many believe, may it be taken under an emergency situation. It would not be possible to withdraw such a decision once taken i.e. if dissolution test alone can provide quality assessment of the products, then why would BE studies be needed and required on what basis especially when BE studies are known to be irrelevant (link).
- This new development is a gift from heaven for the underdeveloped countries where because of lack of BE studies, products and their manufacturing have always been labelled inferior. However, with the requirement of dissolution testing only, everyone can manufacture and promote (for local and/or international markets) their quality products with confidence.
Keep these thoughts in mind and proceed accordingly.
Bye Bye – Bioequivalence testing? Long live drug dissolution testing!
Bye Bye – Bioequivalence testing? Long live drug dissolution testing!
Recently FDA provided 1- and 2-pager guidance documents for chloroquine and hydroxychloroquine, respectively (link).
The most interesting part is that one can get product approval based on dissolution testing alone. This is what has recently been suggested in one of my recent published articles i.e. products (“quality”) assessment can easily and accurately be established with drug dissolution testing alone (link). There is really no need of conducting bioequivalence (BE) assessments. These (BE) assessments procedures have never been validated of the intended purpose. In fact BE are scientifically invalid and can provide false conclusions and assurance about product quality. In addition, such testing exposes healthy human volunteers to highly potent chemicals under the disguise of medicine development.
On the other hand, switching to dissolution testing alone using currently recommended USP apparatuses is not valid either, at least scientifically. The recommended apparatuses are non-GMP compliant and can provide false and irrelevant results because of their intrinsic design and operation problems. Simpler and scientifically valid options are available and could be used (link).
Is Coronavirus really causing abnormally higher number of deaths?
Is Coronavirus really causing abnormally higher number of deaths?
Mortality in the United States, 2018 (as of January 2020, link).
“The age-adjusted death rate decreased by 1.1% from 731.9 deaths per 100,000 standard population in 2017 to 723.6 in 2018.” i.e. death rate is about 0.7236%
For the USA, having population of 331 million (link), normal/standard death (attrition) rate should be 199,593 deaths/month. Now compare this number with the reported number of deaths caused by Coronavirus pandemic, which are 21,435 in about a month’s time as of April 12, 2020 (link) which is far less than normal/standard death (attrition) rate.
The death rate, therefore, does not appear to support the thesis that the pandemic is killing people with abnormally high numbers.
My recently published book chapter (details below) …
My recently published book chapter (details below) …
… provides in depth discussion on establishing and monitoring quality of pharmaceutical products in particular tablet and capsule. I hope you will find the chapter useful.
Chapter title: “Quality” of pharmaceutical products for human use – underlying concepts and required practices, published in
Drug Delivery Trends: Expectations And Realities Of Multifunctional Drug Delivery Systems Volume 3 Edited by Ranjita Shegokar, PhD., Available from Amazon (March 18, 2020), link.
Authorities (including FDA) and pharmacopeias (including USP) never establish quality of products!
Authorities (including FDA) and pharmacopeias (including USP) never establish quality of products!
Reasons:
(1) They do not define quality of the products, hence it cannot be measured and/or established (link).
(2) Suggested methods and procedures lack scientifically relevancy and validity (link)
(3) GMP practices, including inspections, are about operation of manufacturing not per se reflection of products quality (link).
Can Regulatory And Pharmacopeial Compliance Practices Establish Quality?
Similarity Factor (F2) – false and illusionary “statistics”!
Similarity Factor (F2) – false and illusionary “statistics”!
In case, anyone is looking for sophisticated “statistical” jugglery, F2 provides an excellent example of thoroughly confusing people and science. Everyone has to use it (compliance requirement), as it is suggested in the regulatory (FDA) guidance documents. This parameter has not been described in statistics and absolutely has no relevance to the assessment of quality of manufactured products (tablet/capsule). It has been developed using drug dissolution data, and then applied to such data, which are invalid and irrelevant to start with. Calculation wise, it is simpler than calculating typical standard deviation more like a skill-test arithmetic exercise often provided at the back of lottery tickets or some sort of promotion or advertisement (link). Now consider this, a 2-day workshop/conference (yes two full days) is organized at the university level to explain and teach about it to make it look like science or statistics!!! (link or here). There certainly is a serious disconnect here between science and its practice at the regulatory level which requires attention.
Possible interpretation of the FDA response to my Citizen Petition – a positive and encouraging development
Possible interpretation of the FDA response to my Citizen Petition – a positive and encouraging development
I submitted a Citizen Petition to the FDA, on October 1, 2018, requesting withdrawal of its guidance documents and related recommendations concerning assessments of drug dissolution characteristics of pharmaceutical products such as tablet and capsule (link).
The FDA responded, on April 1, 2019 on an interim basis, stating that FDA has been unable to reach a decision on the petition because it raises complex issues requiring extensive review and analysis by Agency officials (see here) or @ the FDA site (link).
My view is that a decision has already been made in support of the Petition (i.e. withdrawal of the guidance documents); however, FDA requires time for its implementation. My reasoning is as follow:
The Petition in fact has two parts; Part 1 (Rationale) – requiring the use of currently recommended dissolution testers (USP Paddle/Basket) which have never been validated and/or qualified for the intended purposes i.e. relevance to assessment of products dissolution in humans. Further, if a (blinded) product sample is given to an analyst, he/she would not be able to determine its dissolution characteristics, which makes the test scientifically invalid and practically useless. Part 2 – (Guidance withdrawal) considering the lack of validity of the apparatuses and testing as described in Part 1, the guidance documents become null and void as these depend on the use of non-validated apparatuses, hence their withdrawal was requested.
To dismiss the Petition, all FDA had to do was to provide: an evidence (link, reference and/or laboratory document) demonstrating that apparatuses are validated/qualified for the intended use. This should have required practically no time. Secondly, if an example was not available already for the analysis of a blinded sample, then all it would have taken the Agency couple of days (maximum) to run an experiment to establish dissolution characteristics of the product. As the testers are not qualified or validated, obviously, testing of a (blinded) sample is not possible. Thus, Petition could not be dismissed.
Therefore, in my view, FDA is considering withdrawing the guidance documents. This should not require extended time period as well. However, considering complexity of the issue (as noted in the response as well) such as meeting procedural formalities, along with associated adjustments, may require extra time which is understandable. This may easily take many months.
If my interpretation is correct, then it becomes important to inform the scientific and manufacturing communities promptly about the situation so that they should be cautious and avoid using the non-validated dissolution testers and methods. In addition, it will provide an opportunity to explore other options for addressing the issues, perhaps submitting ideas to the authorities as well for moving forward – noting that at present no one is determining, or can determine, valid or useful dissolution results of any product (1, 2).
I look forward to a prompt resolution of the issue by removing the non-validated/non-qualified (thus non-GMP) dissolution testers and tests, along with the associated guidance documents, from the regulatory system leading to simpler, efficient and science-based approaches for the assessment and monitoring quality of the pharmaceutical products.
Strange regulatory and pharmacopeial requirement for establishing quality of pharmaceutical products!
Strange regulatory and pharmacopeial requirement for establishing quality of pharmaceutical products!
Requiring the use of drug dissolution testers, which do not (and cannot) determine dissolution characteristics of any product (tablet and capsule in particular). Amazing fact is that these are the only ones mostly recommended and accepted by the authorities, even sometimes as substitute for clinical (bioequivalence) assessments. Why? How does it make sense – scientific or otherwise?
Some links for further details:
(1) Note that no one can determine, or has determined, dissolution characteristics of any product using the currently suggested apparatuses and/or methods. It has all been an illusion! (link)
(2) Bio-waivers! (link)
(3) Pharmacopeial Dissolution Testers (link)
Selecting medium for drug dissolution testing: Please pay attention to the principles of science and the laws of nature
Selecting medium for drug dissolution testing: Please pay attention to the principles of science and the laws of nature
Considering solubility characteristics of a drug in the stomach (i.e., pH range of 1 to 3) are pretty much useless from the perspective of absorption of a drug. Even if a drug gets dissolved in the stomach, it will be precipitated out in the intestine if it has lower solubility at a higher pH.
For absorption purposes a drug must dissolve, not necessarily completely but in some quantity, in the intestine where pH ranges from 4.5 to 7. Drugs get absorbed in steps in the intestine by continuous extraction process thus complete dissolution of drugs, at any given time (so called “sink condition”) is not necessary.
Moreover, dissolution characteristics are not usually determined for a drug – dissolution tests are conducted to evaluate products. The choice of medium is linked to the physiological environment of the GI tract not to the drugs or products. Please pay attention to the principles of science and the laws of nature. For further detail please follow the link (link).
Simulation and modelling practices for establishing quality of pharmaceutical products – valid intentions but invalid outcomes
Simulation and modelling practices for establishing quality of pharmaceutical products – valid intentions but invalid outcomes
Dear experts:
In the area of simulation and modelling, including developers of commercial software for such, note that I may not be able to argue with you regarding your methodologies of data analysis, modelling and/or simulation aspects as this is not my area of expertise; however, I know with certainty that you would require valid and accurate data for your analysis purposes. The difficulty is that you would not have access to such valid and accurate data at least for the evaluation of tablet or capsule products for the prediction of plasma drug levels or profiles. That is, in vitro drug dissolution results which represent or simulate in vivo dissolution and by extension plasma drug levels or profiles. You would require such data to validate your simulation or modelling outcome at least for the product development and manufacturing stages. Unfortunately, no one, at present, is generating, or can generate, valid in vitro dissolution data, thus your efforts of conducting simulation/modelling are regrettably of no use and would not help the industry, regulatory authorities or anyone else. Please, do not make claims of the successes and usefulness of such exercises.
One of the main reasons for not being able to obtain valid in vitro dissolution or drug release data is that the recommended and required (e.g. from USP and FDA) dissolution testers for such purposes have never been shown to provide valid and accurate dissolution results i.e. these testers have never been validated for their intended use or purpose. Vendors/manufacturers make extraordinary efforts and take pride in providing “compliant” testers i.e. meeting or exceeding “physical or fixed” specifications according to the pharmacopeial (such as USP) requirements; however, unable to validate the testers as dissolution testers. For example, no vendor, at present, can provide you valid in vitro dissolution results if given a blinded sample of a tablet/capsule product. Therefore, in this respect claims made by the vendors are also not accurate that they are selling or manufacturing dissolution testers. At best, the only claim they can, or should, make is that they are selling simple stirrers. Perhaps more disturbing is the fact that these stirrers when used as required for dissolution evaluations, because of their design and operation limitations and flaws, cannot provide valid and accurate dissolution results which is documented extensively in the literature.
In short, please use and promote the simulation and modelling techniques with care and certainly use extra caution in making claims for such about the future expectations and successes.
Quality of pharmaceutical products: “Regulatory Science” – its illusions, obstacles and a potential solution.
Quality of pharmaceutical products: “Regulatory Science” – its illusions, obstacles and a potential solution.
“Regulatory Science” is a term often used to describe practices of national, sometime international, bodies to establish and monitor quality of pharmaceutical products such as tablet and capsule, which would include safety and efficacy aspects as well. A clear description of the term “Regulatory Science” appears to be lacking. In practice it may be considered as a practice of setting standards (specifications) and protocols for describing and establishing quality of products available on the market for human use. The underlying concepts for setting standards/specifications and protocols usually come from the fundamental principles and laws of sciences, engineering and mathematics such as biology, chemistry, manufacturing and statistics. “Regulatory Science” uses these scientific principles to set specifications and protocols, rather than generating new scientific knowledge which is generated under the auspices of a specific scientific and/or engineering discipline. Therefore, regulatory bodies hardly ever generate new scientific knowledge but use it to generate specifications and standard procedures for implementation for the public good. The authorities also get the mandate to enforce the developed and suggested specifications and protocols – as these are intended to be followed. This mandate of enforcement results in the term “compliance” i.e. industry must adhere (“compliant”) to the standards and protocols for their manufactured products to be approved for marketing.
The role of the authorities may be explained with an analogy of a distribution company (e.g. Amazon, eBay, Costco) which acts as a go-between a manufacturer and its consumers. These distributors hardly ever manufacture or develop any of the listed products they sell; however, they provide assurance about the quality of the products and their appropriate delivery to the consumers or customers meeting well described quality standards/specifications. The distributors’ role is limited only to providing or transferring quality products from manufacturers to consumers nothing more. If a product has a fault or issue of quality/compliance, discovered through distributor’s own internal audit or by third party, the particular supplier or manufacture of the item is informed. The manufacturer has to deal with the issue. The distributor does not start addressing the issue or advising the manufacturer as to how to fix the manufacturing problem as they would lack needed expertise. If the manufacturer is unable to resolve the issue then the product has to be taken of the market.
Considering the pharmaceutical product manufacturing area, the regulatory authorities’ mandate is that of a go-between, like a distributor as described above, to ensure that the manufactured products available on the market must be of quality. For this, authorities have to define a “quality product” and then set its “quality attributes”, along with measurable specifications, in collaboration with the manufacturers. Unfortunately, the authorities do not provide definition/criteria for the “quality product” but expect that the manufacturers must provide quality products – obviously they can’t! Therefore, by default manufacturers become guilty of not providing quality products and thus they need to be “corrected or fixed”.
With this presumed lack of trust or capability of the manufacturers came the regulatory guidance and inspection based system to guide and/or advise the manufactures as to how to manufacturer quality products. Therefore, mandated responsibilities of the authorities have completely deviated (metamorphosed) from establishing and monitoring quality of the products to the quality of the manufacturing. Considering the lack of competencies of the authorities in manufacturing, as they never manufacture or develop any product like the above mentioned distributors, understandably they would be unable to advise the industry appropriately and correctly especially in the absence of a definition of quality products and their attributes. Rather than providing appropriate definition and standards for quality products which industry should follow authorities frequently start a new fad of “science based guidance” documentation such as SUPAC, IVIVC, QbD, ICH, cGMP, PAT, inspections etc., commonly even violating well-established scientific principles, for enforcing a new set of requirements for manufacturing. This practice has resulted in perpetual cycles of more guidance documents, reorganizations, rebranding and policing activities turning the authorities from facilitators to bullies blaming and/or punishing the industry for not following quality standards and practices. It is impossible for the industry to follow arbitrary protocols and/or employing non-validated tests and procedures, as recommended and required by the authorities, to provide a scientifically valid outcome and by extension quality products. This has put enormous burden, administrative and financial, on the industry and society in general – without improvement in manufacturing or availability of quality products and/or addition of any other value.
Therefore, to resolve this issue the regulatory authorities, world-wide, should reconsider focusing their role as the standards setting organizations for the products and not that of providing guidance to the industry as how to manufacture products and market these to the public.
Some relevant links which would be useful read in this regard:
(1) http://www.drug-dissolution-testing.com/?p=3040
(2) http://www.drug-dissolution-testing.com/?p=3047
Time to rescind the regulatory requirements of bioequivalence evaluations and the current pharmacopeial drug dissolution practices as these do not provide quality assessment of pharmaceutical products
Time to rescind the regulatory requirements of bioequivalence evaluations and the current pharmacopeial drug dissolution practices as these do not provide quality assessment of pharmaceutical products
Considering the non-specificity, because of confounded variabilities from the physiological system, drug release assessment of pharmaceutical products (tablet/capsule) for which this test is conducted, the bioequivalence test becomes a scientifically and statistically in-valid practice. See here for further discussion on the topic (1,2).
An in vitro drug release test, commonly known as drug dissolution test, which by its nature avoids the above mentioned non-specificity, provides a better alternative for assessing drug release characteristics of the products thus their quality. Pharmacopeias worldwide recommend this test. Unfortunately the recommended testers suffer a serious design problem thus providing irrelevant and unpredictable results not reflecting product quality or lack of it (3,4). In short, the drug dissolution tests as currently recommended are based on non-qualified and/or non-validated testers, hence results from the testing cannot be relied upon. Therefore, their use is to be discontinued as well.
As a solution, a simple revised dissolution testing approach has been suggested which would provide superior drug release evaluation thus quality of the products for human use (5, 6). In addition, as it is an in vitro technique, the test can be conducted without the use of human subjects avoiding unnecessary risk to participating healthy volunteers and/or patients. The suggested approach not only provides scientifically valid method for assessing quality of the pharmaceutical products but also would give much needed flexibility to pharmaceutical industry for innovation to bring out products faster, and with a reduced price, into the market.
Quality generic products without bioequivalence (BE) assessment – a simple and practical approach!
Quality generic products without bioequivalence (BE) assessment – a simple and practical approach!
Considering the weakness (non-specificity) of BE assessments it is suggested that in vitro drug dissolution/release testing would provide a better alternative to establish quality of pharmaceutical products such as tablet and capsule. It is argued that the use of in vitro dissolution test should be the method of choice for developing and monitoring improved or better quality generic products because BE assessment focuses only on equivalence and not on the improvement of the product quality. Other significant advantages of using an appropriate in vitro dissolution test in lieu of BE assessment are described..
For further detailed explanation please follow the link.
Pharmaceutical products quality and bioequivalence assessments – what a waste and needless use of human subjects!
Pharmaceutical products quality and bioequivalence assessments – what a waste and needless use of human subjects!
A bioequivalence study is conducted in humans to establish that two or more products are capable of providing same/similar blood/plasma drug levels. Underlying assumption is that if the products provide same plasma drug levels then their therapeutic effects would be the same as well, thus would allow interchangeability of the products such as the generics.
Therefore, for all practical purposes the bioequivalence assessment may be considered as a typical analytical chemistry test where the assessment is based on determining plasma levels. For conducting an appropriate and accurate analytical test, the test must follow some fundamental principles of analytical tests such as specificity and its validation (accuracy, precision and reproducibility). A test cannot be validated if it is not specific.
In this regard, a bioequivalence test is a non-specific test as plasma drug levels include (confounded) variabilities from stomach emptying/motility and liver metabolism of the drug – independent of the product characteristics. Therefore, caution is warranted in establishing quality of the test products based on the bio-equivalence test.
For further detailed explanation please follow the link.
Consumers and patients must wait, and suffer, for the availability of quality pharmaceutical products such as tablet/capsule as well as their genuine and affordable prices. The reason may surprise you!
Consumers and patients must wait, and suffer, for the availability of quality pharmaceutical products such as tablet/capsule as well as their genuine and affordable prices. The reason may surprise you!
It is important to note that at present availability of the pharmaceutical products such as tablet and capsule is heavily regulated, more accurately controlled, by the regulatory authorities worldwide. Manufacturers and suppliers have to follow extensive suites of protocols (national and/or international) to get their products approved for marketing. These protocols are often described by different names such as regulations, guidelines, standards etc. The manufacturers have to be in compliance with these protocols literally to the letter, which are mostly arbitrary in nature. Thus, in practical terms contrary to popular belief, there is limited or no room for deviation, simplification and/or innovation from these protocols at least from the manufacturers’ side.
In simple terms, these protocols may be considered as formats for data/results presentations, may these be for the product development or manufacturing – promoted as regulatory science. However, unfortunately, these are administrative and procedural requirements, not the practice and/or requirement of the science. The underlying “science” remains based on traditional practices and assumptions, more accurately may be considered as rituals. Therefore, with the passage of time and the introduction of extensive sets of standards and requirements, the burden of adhering to these regulatory formats (“guidelines”) has become increasingly frustrating, time consuming and financially challenging for the both, authorities and the manufacturers, without any added value to the product quality and/or benefit to the users.
In addressing these challenges, manufacturer bashing approaches (implied or explicit) are common and fashionable, often criticizing lack of their integrity and competencies. This approach certainly appears to be a deviation away from the regulatory mandate or requirements which is establishing and monitoring quality of the products and not that of assessing and criticizing manufacturing ability or capacity. Regulators’ and their associates should be able to establish if the manufactured products, at the consuming stage, are of the required quality, and by extension, safe and efficacious. However, they can’t at present – thus deviation from their mandated objective!
There are two reasons for this regulatory shortcoming: (1) Regulatory authorities have never defined required quality, and its associated parameter, for the product assessments. In fact, it could be argued that it is unknown to them. (2) Authorities require and enforce a large array of flawed product testing requirements for compliance purposes without their validations and relevance. As these requirements lack scientific credibility and validity, anybody, not just the manufacturers, would have difficulty in meeting or will be unable to meet the current regulatory requirements and expectations. For a more technical description of this aspect please consider viewing the links provided below.
Therefore, there is a clear need for re-evaluating the practice of setting regulatory standards and requirements starting with the definition of a quality product followed by the use of scientifically/GMP valid instruments and procedures. Otherwise, it is impossible for the manufacturers to produce quality products, and for the regulators developing and implementing appropriate guidelines and standards for product evaluation.
Some suggestions are provided to address these issues, and it is sincerely hoped that authorities will give consideration to these thoughts.
For further reading:
(1) http://www.drug-dissolution-testing.com/?p=3022
(2) http://www.drug-dissolution-testing.com/?p=3007
(3) http://www.drug-dissolution-testing.com/?p=2956
(4) http://www.drug-dissolution-testing.com/?p=3037
(5) http://www.drug-dissolution-testing.com/?p=2922
Regulatory compliance is more appropriate description than QC/QA
Regulatory compliance is more appropriate description than QC/QA
It is important to note that at present pharmaceutical laboratories are operating under non-GLP/GMP conditions, in particular for the assessment of solid oral dosage forms such as tablet and capsule products. This is surprising that such negligence has been going on unchecked. This deficiency needs to be corrected so that facilities can be considered as QC/QA laboratories to provide relevant and accurate quality characteristics of the products. For further details, please follow the links (1, 2, 3).
Drug Dissolution Testers (Paddle/Basket)
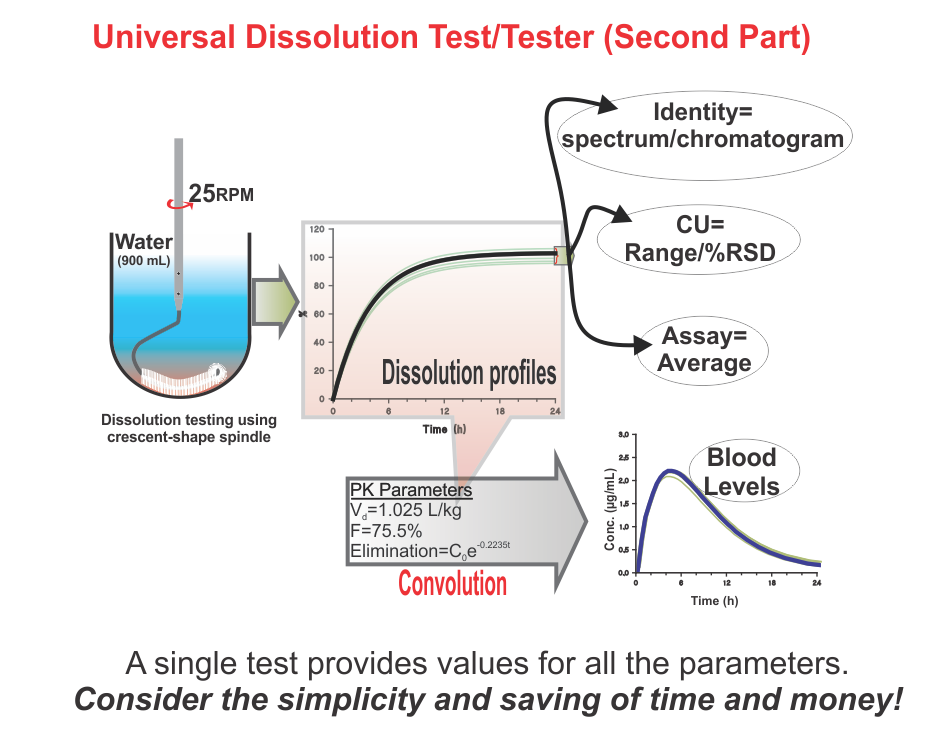
Universal Dissolution Test/Tester – Second Part
Suggestions of developing and validating dissolution methods using compendial apparatuses, which are not qualified and validated, are simply promoting false science and useless practice!
Suggestions of developing and validating dissolution methods using compendial apparatuses, which are not qualified and validated, are simply promoting false science and useless practice!
The above title is self-explanatory, clear and says it all.
People have to be careful when offered help in developing and/or validating dissolution methods based on non-validated apparatuses (e.g. paddle/basket) and/or experimental conditions. The results obtained would not be of any use, even for QC purposes, no matter how they are presented.
The following links may be of further help in this regard.
(1) If one cannot determine dissolution characteristics of a product then how would one be able to establish its quality or bio-relevance? A serious flaw of current practices! (link).
(2) De-aeration of a medium and vibration free environment – perfect attention deflectors (link).
(3) Note that no one can determine, or has determined, dissolution characteristics of any product using the currently suggested apparatuses and/or methods. It has all been an illusion! (link).
(4) Assessing drug dissolution characteristics using product dependent methods is simply unscientific and invalid practice. (link).
(5) Dissolution method development: Perhaps the most wasteful of all the current practices! (link).
(6) Current practices of drug dissolution testing using paddle/basket apparatuses – A complete waste of time! (link).
(7) Promotion of simplicity of paddle/basket apparatuses – A marketing gimmick for scientifically useless and non-validated apparatuses (link).
(8) Drug Dissolution Testing – A serious concern! (link).
(9) Drug dissolution testing: Limitations of current practices and requirements (link).
(10)Dissolution Apparatuses: Compliant vs Qualified and Validated (link).
(11)Costly mistake formulators/analysts often make i.e. developing a product dependent dissolution test (link).
(12)Apparatus Calibration or Performance Verification: Misleading Conclusions and False Comfort (link).
Note that no one can determine, or has determined, dissolution characteristics of any product using the currently suggested apparatuses and/or methods. It has all been an illusion!
Note that no one can determine, or has determined, dissolution characteristics of any product using the currently suggested apparatuses and/or methods. It has all been an illusion!
The purpose of a dissolution test is to determine or establish drug dissolution or release characteristics of a product, in particular tablet or capsule. To determine dissolution characteristics, or any other characteristic in general, one would require a method and/or tester or apparatus. Prior to its use, it must be established that the method/tester is capable of providing an expected outcome, i.e. in this case the method/tester is capable of providing dissolution characteristics of a (tablet/capsule) product. In other words, the dissolution method/tester should be qualified and validated.
Unfortunately, at present, suggested and commonly used dissolution testers have never been shown as qualified and validated dissolution testers. Therefore, reported results, and by extension conclusions drawn from these results, are of limited or no relevance or use. It is all an illusionary science and interpretation of data/results.
The crescent shape spindle has been proposed to address this present-day difficulty. The use of the spindle provides an ability to test products using a common, simple testing and product-independent approach.