In a recent article titled “Evaluation of In-Situ Fiber Optics Dissolution Method for Compound A Extended Release Tablets” published in the March/April issue of American Pharmaceutical Reviews (Link), the authors described the usefulness of the technique for dissolution testing. The usefulness is described based on method validation parameters such as specificity, linearity, accuracy/recovery, precision, robustness etc.
To me the precision is the most important parameter as almost all the other parameters depend on it, i.e., if the precision is not acceptable then the other method validation parameters will also reflect poorly, thus the method itself.
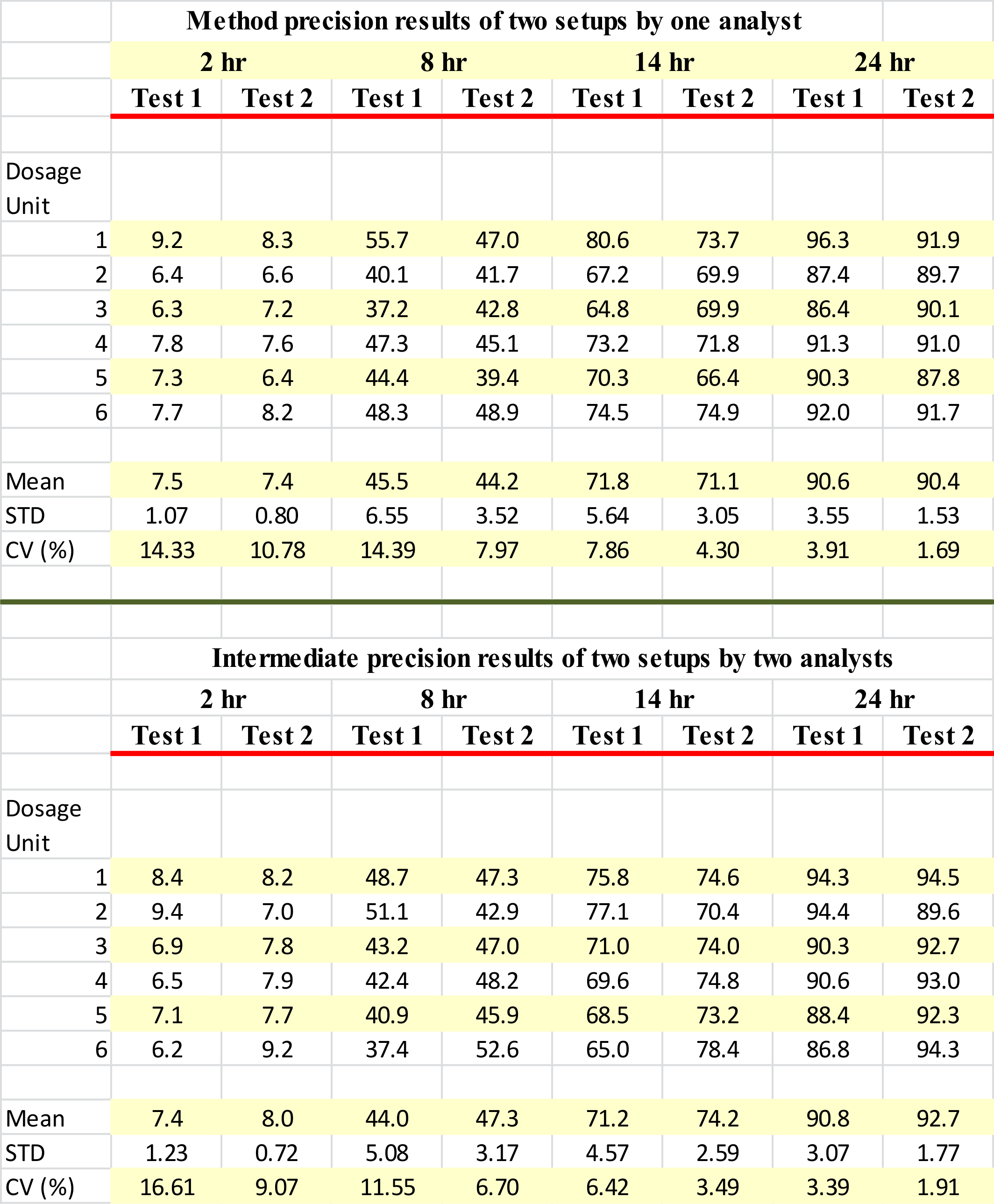
It is very well accepted and common practice to describe the precision of a method based on Relative Standard Deviation (RSD) or Coefficient of Variations (CV) in percentages. However, in the article (method and intermediate precisions, Tables 5 and 6) authors reported no %RSD (CV%) values. Therefore, the question is how is the precision of the method established? Moreover, why would the authors omit the precision (%RSD) values?
The reason, in my opinion, is that the method is not as precise a method as one would like to see. The underlying method here is drug dissolution method which is well known for its high variability in results. Therefore, the use of a detection/quantitation method, whether it is a simple UV-based or in situ fiber optics-based, to evaluate method precision will not help but would make the detector look bad as well. It is a similar situation, where drug dissolution results are reported based on individual (Q-) values, but without associated precision (%RSD values), because one can never get acceptable precision from a dissolution test using paddle and basket apparatuses.
In my opinion, authors should have used only the last dissolution sampling time to describe the usefulness and precision of the in situ fiber optics approach. The reason being, given sufficient time, which is 24 hours in this case, dissolution testing would have been completed. The variation due the dissolution testing would be eliminated and thus would not be a contributing factor. Therefore, the observed precision (%RSD), as shown in the table below, would be true reflection of the quantitation method, which is quite acceptable, less than 4%. Otherwise, %RSD of the method is as high as 16.6% from a single batch and can be even higher for inter-batch and/or inter-lab situations.
In short, for describing the precision of a method (dissolution) or technique (in situ fiber optics), one should provide %RSD (CV%) values. These values should reflect the property of the desired underlying method or technique.