-
IVIVC: (In Vitro-In Vivo Correlation).
This is the most commonly referred terminology and appears to originate from US FDA guidances. It define the IVIVC as, “A predictive mathematical model describing a relationship between an in vitro property of a dosage form (usually the rate or extent of drug dissolution or release) and a relevant in vivo response, e.g., plasma drug concentration or amount of drug absorbed”. Common interpretation: Usually a point-to-point relationship is to be established between the in-vivo and in-vitro parameters of the same time points (e.g., in-vivo fraction drug absorbed vs in-vitro fraction drug dissolved) by applying a mathematical technique of deconvolution. Practical limitations: Both in vitro and in vivo data are required for the same product. The procedure does not provide predictability but reflects relationship between in vitro (dissolution) and in vivo (fraction absorbed/dissolved), if successful. As results are compared/related using a single product, the approach may not be used for the comparison of products, for their formulation/manufacturing attributes.
-
IVIVM: (In Vitro-In Vivo Matching).
I often use this terminology and discussed it in some of my publications. IVIVM reflects an unconvincing, perhaps misleading, interpretation and practice of IVIVC. This would require one or more products having different in vivo (blood drug concentration-time) profiles and then a number of in vitro dissolution profiles, using different sets of experimental conditions. If one set of experimental conditions provides a matched ranking between dissolution and in vivo profiles, then it is considered as achieving IVIVC. The dissolution test would be called as bio-relevant. If none of the prior dissolution methods provide such matching, then a new set of experimental conditions may also be developed to match the ranking. It is clear to see that this approach seeks to match, thus would NOT reflect a relationship or predictability aspect, which is the requirement of an IVIVC.
- IVIVP: (In Vitro to In Vivo Profiling).
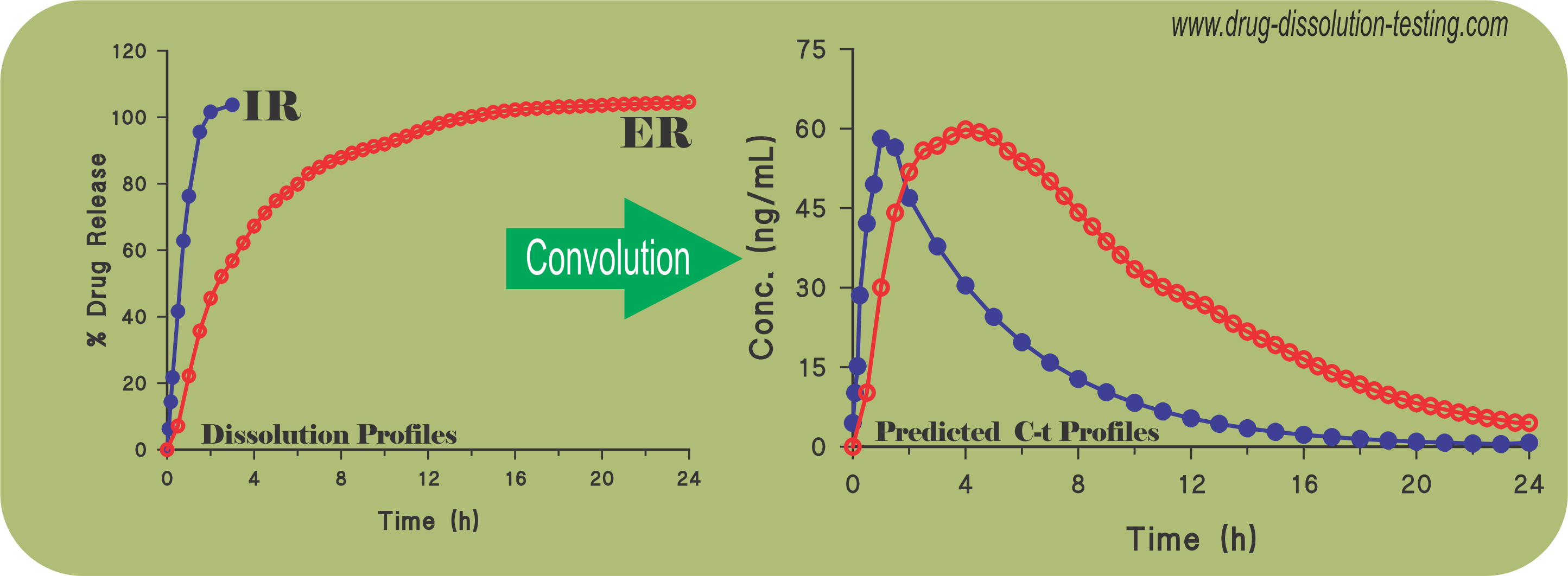
Considering limitations in defining or interpreting IVIVC, a more appropriate and direct definition and interpretation is desirable. For this, one needs a clear and practical objective for IVIVC, which is to link or relate the in vitro (dissolution) and in vivo (blood drug concentration-time, or C-t) profiles. In this regard, the main purpose to conduct a dissolution test is to establish a dissolution profile and then predict/determine a C-t profile from it to assess potential in vivo characteristics of the test product. Therefore, it can be said that in reality the purpose of commonly referred practices of IVIVC is to transfer a dissolution (in vitro) to a C-t (in vivo) profile, or simply in vitro-to-in vivo profiling. The mathematical technique to transfer in vitro profile to in vivo profile is known as convolution. Convolution is relatively simpler than de-convolution as the former can be applied using simple spreadsheet software, e.g., MS Excel. For further discussion of the subject and detailed methodology to achieve IVIVP please see the links (1, 2).
Drug Dissolution Testing
- For Simple and Practical Ideas -