Product development stage: What this really means in simple terminology is the stage where a product (formulation + manufacturing process) is developed to show that it is capable of releasing (dissolution) the drug and providing desired drug levels in humans. The drug release characteristics of the product are usually established based on human studies which are commonly known as bioavailability/bioequivalence (BA/BE) studies. However, one requires a simpler in vitro method to screen test products (especially multiple combinations of formulations) to select some (usually one or two) for BA/BE studies.
Drug dissolution test: This is the in vitro test which is used for this purpose i.e., to evaluate potential release characteristics of different products (or formulations). It is, therefore, very important to note that at this stage a formulator must have access to a dissolution method which is capable of reflecting potential in vivo drug release (dissolution) characteristics in humans. This method must already be developed and validated using other well characterized product(s) for human use. In the literature, it is often described that a specific dissolution method be developed at this stage for the particular test drug/product. However, such a practice is scientifically invalid, as a method can only be developed using a product with well established dissolution characteristics. At the product development stage a dissolution test is applied not developed. This is a very important concept, often over looked and should, therefore, be kept in mind.
The next step: Preparing a variety of products (or formulations) and obtaining in vitro dissolution characteristics of these products would not be valuable or useful until it is shown what type of drug levels these products/dissolution results will provide in humans. It is important to note that obtaining similar or different dissolution characteristics for different formulations are usually of limited use. One has to demonstrate what would be the expected drug levels in humans from the test formulations. As stated above, the exercise of product development is to develop a product to achieve desired drug levels in humans. Therefore, there must be a technique to convert these dissolution results to potential drug levels in humans. Such a technique, known as convolution, is described in the literature (link) which may be used to transfer dissolution results to blood levels in humans. 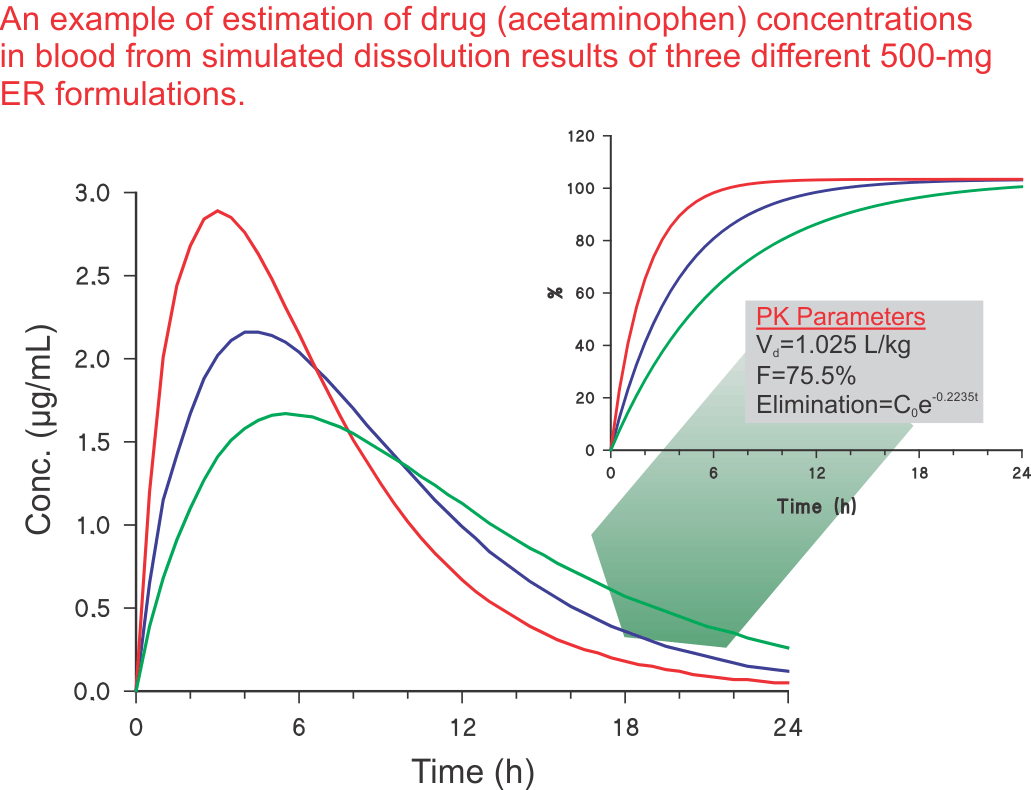
For the purpose of an illustration, an example is provided to reflect the estimation of acetaminophen blood levels from dissolution results for a 500-mg extended release product (see Figure). The methodology of convolution is described in detail in one of the publications (link).
Once a desired drug’s levels profile is achieved then the formulator may use the product for an in vivo BA/BE study to finalize the development of a product.
If one follows the approach described here, not only will it provide a powerful screening method for selecting products for BA/BE testing, but also significantly simplifies the product development stage.
