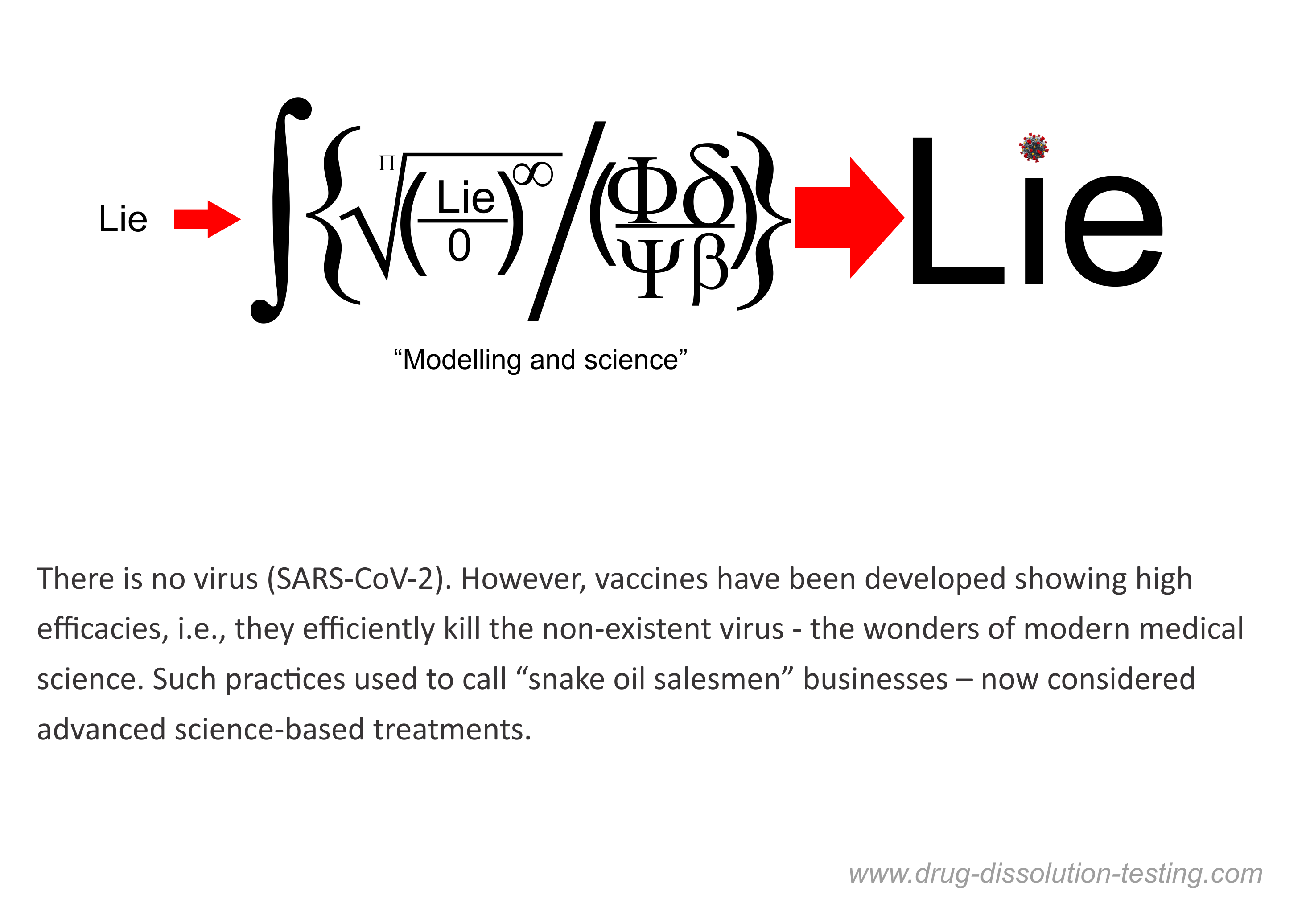
Vaccines: Full Approval?
How could the FDA recent letter, Letter of Authorization [1], be considered FULL APPROVAL when the document clearly and repeatedly uses the term EUA (Emergency Use Authorization)? The document ends with a statement,
“This EUA will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic is terminated under Section 564(b)(2) of the Act or the EUA is revoked under Section 564(g) of the Act.” Continue here
Drug Dissolution Testing: Useful Lists
Useful Lists
Please select a sub-menu
Drug Dissolution Testing
Should FDA, and other authorities, approve the SARS-CoV-2/COVID-19 vaccines? – A scientific perspective
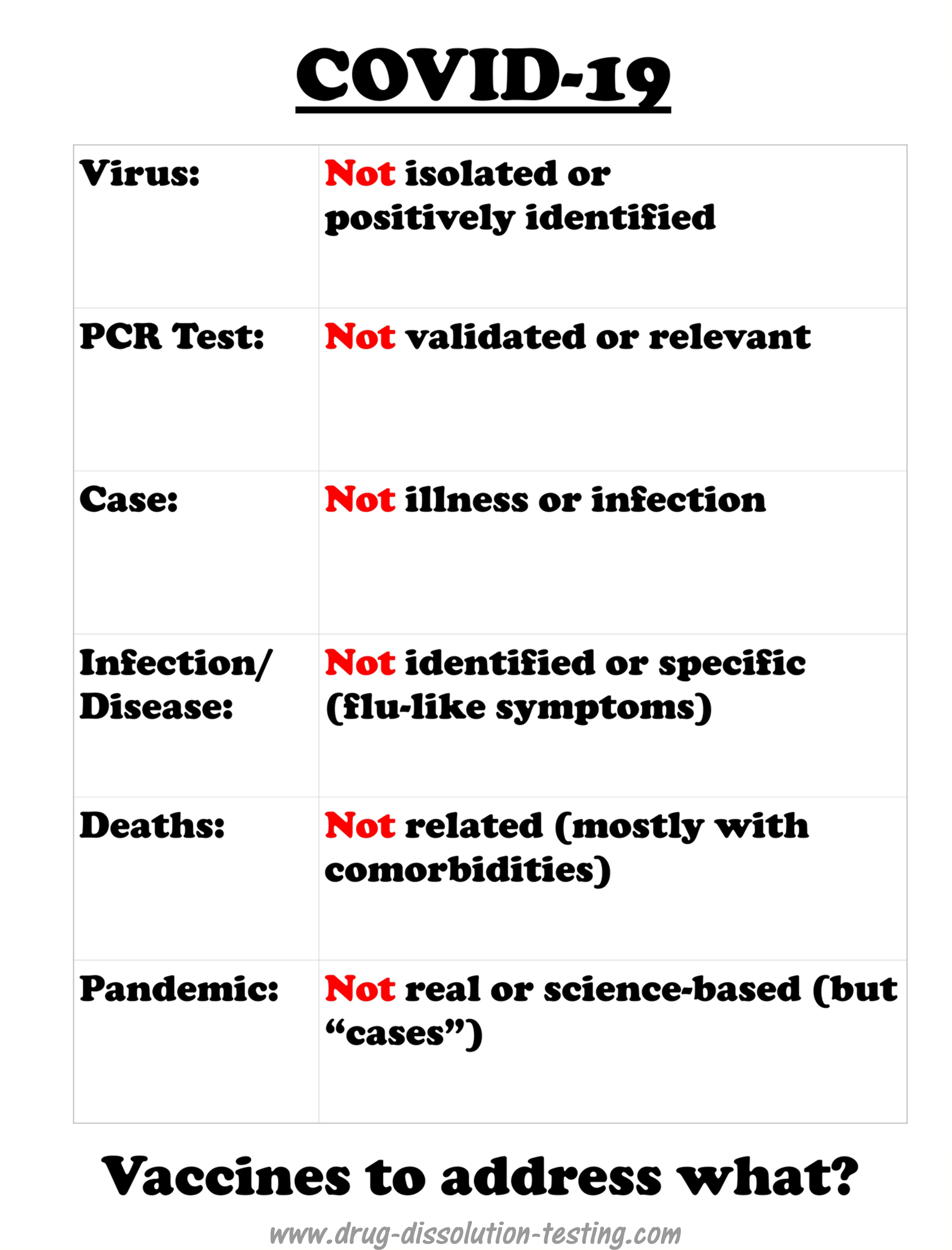
COVID-19 is a recently labeled infectious disease which is presumably caused by a novel coronavirus labeled as SARS-CoV-2.
It is important to note that COVID-19 is not based on any defined and specific symptoms but common and general flu-like potentially treatable with antibiotic regimens [1]. However, medical experts and regulatory authorities, in particular FDA, have adopted an official position that illness is because of a viral infection caused by SARS-CoV-2. Being a viral disease led to a policy decision that a vaccine is needed for its treatment that is to be developed. The pharmaceutical industry has made great efforts in collaboration with the authorities to develop vaccines quickly. There have been media reports that some vaccines are at a late development stage and ready to be submitted to the FDA for marketing approval.
Continue here
What does a positive coronavirus test really mean?
One thing is for sure that the test does not show the presence of the virus. Anyone who says otherwise is misinformed or lying. At present, there is no test available to test the coronavirus.
The test, which is usually conducted using swab samples, is called the PCR test. The PCR test does not test the virus, but a long chain chemical compound commonly known by its fancy name DNA or RNA. Therefore, the test is a chemical test to monitor a chemical compound (DNA or RNA) or its part by its nature. With a positive test result, it is assumed that the RNA is from the coronavirus. However, because no one has seen or isolated actual coronavirus, it is impossible to establish if the RNA (or its part) is from the virus. The positive test results could be from any or many sources, including regular seasonal, live or dead, flu-virus, or its debris.
Also, scientifically speaking, before any test is accepted for its intended use, it must be validated to show that it is specific and accurate. However, the current PCR test could not be validated because it would require coronavirus itself and its RNA that is not available. Therefore, the PCR test becomes invalid or irrelevant and unpredictable.
So to the question, “What does a positive coronavirus test really mean?” The answer is NOTHING! For a more detailed discussion on the topic, please follow the link..
(Revised for editorial changes on November 23, 2020).
CDC virus testing and isolation claims for SARS-CoV-2 and COVID-19: Non-scientific and pure illusion!
A few days ago I provided critical comments on a publication from Australia (University of Melbourne) which claimed isolation and identification of SARS-CoV-2 virus [1]. I suggested that claims were not supported by scientific evidence and logic.
The present article critically evaluates a similar claim from America’s Center for Disease Control (CDC) in a publication [2] entitled: Continue here
Isolation and characterization of the virus (SARS-CoV-2)
It is both logical and common sense to expect that if a particular material is claimed to exist then its presence must be established using valid and well-recognised practices and laws of science. For example, if it is suggested that a certain geographical area may provide a significant amount of mineral such as gold or oil then that mineral must be extracted, isolated and characterized before proceeding with large scale production for public benefit and commercial gains. The same understanding has to be applied in other areas including the medical and pharmaceutical areas.
At present the world is allegedly in the grip of a serious and wide-spread disease (pandemic) referred to as COVID-19 caused by a virus labelled as SARS-CoV-2. Hence, there is purportedly an urgent need for a treatment for this disease. Commensurately, it is important to note that the medical community has declared with apparent certainty that disease (COVID-19) exists and is caused by the virus SARS-CoV-2. [click here to continue]
Edited and revised for clarity and grammatical improvements on November 5th, 2020.
Drug Dissolution Testing
More bad news concerning virus isolation and PCR test
I have been highlighting for some time that the virus (SARS-CoV-2) has never been isolated or positively identified [1,2,3,4]. Therefore, it cannot be claimed that the virus exists, and by extension, the story of the COVID pandemic cannot be considered science-based or factual.
This idea of non-isolation of the virus has been gaining traction. A recent report further emphasized that the SAR-CoV-2 has not been isolated, along with any other viruses in the coronavirus family [5].
The use of the word “isolate,” with the implied meaning or representation of the term isolation of the virus, misled everyone, including physicians, scientists, experts. They assumed that the virus or viruses are real and have been physically isolated.
The article mentioned [5] above also clearly discredit the PCR test’s relevance and usefulness, as I have been saying for quite some time [6,7,8]. A prestigious international expert on the subject, Dr. Stephen Bustin, is quoted, describing both the arbitrariness of criteria for RNA results and choosing the number of cycles leading to anyone testing positive for COVID. The mentioned article [5] discusses the flaws of PCR tests and methodology for its use as a diagnostic tool.
On the other hand, as I have repeatedly described, the PCR test is a chemical test that has never been validated for its intended use. It is a blatant violation of the fundamental principle of science-based chemical/clinical testing. Such a test can never provide relevant and valid results. Surprisingly, such testing is accepted by the regulatory authorities, including the FDA. The test and its associated results should be withdrawn immediately.
In short, claims of isolation of the virus (SARS-CoV-2) and the PCR test are shown to be scientifically invalid and irrelevant.
(Video) Virus, COVID, pandemic, vaccine, and testing: fiction, not reality or science!
Click on the picture to play the video
If you prefer to read from the text, it is available here (link)
Please donate (by clicking the button below). Thanks.
Did COVID cause “excess deaths?”
COVID-19 is a recently-labeled illness presumably caused by a virus named SARS-CoV-2. The illness is considered contagious, i.e., assuming that the virus spreads from person to person directly or indirectly. It is believed that COVID-19 caused the pandemic resulting in a large number of deaths.
This article reflects an exercise in summarizing the data in seeking a potential trend from COVID-19 deaths to guide addressing the pandemic issue. (Continue here)
Getting out of the coronavirus pandemic – legitimate and scientifically valid approach
People should realize that reducing the Ct (cycle threshold) of the PCR test, as some suggest, may reduce the number of test-positive results. This will certainly help reduce the so-called pandemic—a trickery approach to bring the pandemic under control that never existed in the first place.
However, the fact remains that the PCR test is scientifically invalid, no matter how low Ct value one would set. To have a valid test, it requires to meet four well establish validation criteria: (i) specificity; (2) selectivity; (3) reproducibility; and (4) use of independently characterized reference standard. The first three criteria cannot be met if the reference standard (the #4) is not available. If the claim is that the PCR test monitors the presence or absence of the virus. Then reference virus must be available in its pure form. On the other hand, if virus RNA or its fragment is to be monitored, RNA or its fragment must be available as the reference standard and positively shown to be extracted from the virus (further details here).
Considering that the tests or testing are based on confirming the RNA sequencing only from an aliquot of media/culture/isolate, hence is a valid test, is pure nonsense. Such tests have no relevance to the virus, illness, and pandemic monitoring, and these PCR tests must be stopped immediately and preferably withdrawing all related results/data as false. This will be the legitimate and scientifically valid approach for getting out of the pandemic state.
It is hoped that science will prevail.
COVID cannot spread – with or without a mask or social distancing
COVID-19 is an illness presumably caused by a virus named SARS-CoV-2. The illness is considered contagious, i.e., the virus spreads from person to person directly or indirectly. The virus is commonly viewed as a particle. For it to spread, the particle has to exist.
The problem is that such virus particles do not exist because no one has isolated them or positively identified them (details here).
If something does not exist, how it will transfer from one person to another. It can’t.
Therefore, wearing masks or keeping a distance becomes irrelevant. It is that simple!
PCR testing and the viruses
PCR testing and the viruses
I received an email yesterday asking some questions about the PCR test. Considering that visitors to the blog may also find my answers useful, I share my responses here.
.
Dear …:
Answers to your questions may be found in one of my blog articles (link). Please consider reading it. For further clarity, I am providing answers to the questions below as well (in red letterings). I hope you will find the answers helpful.
.
Q: “If a SARS-CoV-2 virus has not been isolated, when the PCR test comes back positive, what has it positively identified?”
The PCR does not test for viruses, i.e., it is not a test for viruses. A PCR test determines the presence of PRESUMED (imagined) RNA or DNA. Therefore, as the viruses have never been isolated, the authenticity of their association to a specific RNA/DNA cannot be established.
A positive PCR test shows the presence of some RNA/DNA from an unknown source. It could be from many sources, including sometimes claimed to be from healthy subjects. A positive PCR test (often considered as “case”) has no scientific meaning or relevancy to the virus/illness.
.
Q: “Is it an influenza virus or some other virus?”
Again, a PCR test is not a test for influenza or any other virus. It is RNA/DNA test without its established or confirmed link to a specific virus.
.
Q: “In addition, can the PCR test distinguish between viruses?”
Of course not. PCR test is not for viruses, so it cannot differentiate between viruses.
.
Q: These are the two questions I have not been able to locate an answer to, and I would be grateful if you could provide clarification.
The reason for the lack of answers is that the PCR test belongs to the science of (analytical) chemistry. However, it is conducted and interpreted by others without appropriate training and knowledge of the subject. Therefore the PCR testing, as it is conducted now (i.e., for diagnosis purposes), must immediately be stopped as being irrelevant.
Another article which may be of interest in this regard would be (link)
Health experts to the public: Please remain terrified – we desperately need it for our professional survival!
Health experts to the public: Please remain terrified – we desperately need it for our professional survival!
As of April 7, 2021, the Government of Ontario, Canada, like other regions in the world, enforced another lockdown with a stay-home order. The stated reason is that the number of “cases” (not infections, illness, hospitalization, or ICU occupancies) has increased above a magical number, and the projections look worrisome.
Unfortunately, the ongoing somewhat lenient lockdown failed to create enough fear in people to “behave.” Lack of presumed obedience has created a problem for maintaining the people’s “safety” (read people are not showing enough interest or trust for the experimental vaccines). Click here to continue.
COVID: Why are the issues with the virus isolation and PCR testing?
COVID: Why are the issues with the virus isolation and PCR testing?
Isolation and testing belong to analytical (chemistry) subjects. Unfortunately, non-chemistry-related subject experts are doing this work and mistakenly labeled it as isolation and testing. This is where the problem is.
Scientifically speaking, the virus has never been isolated, and by extension, relevant and valid tests cannot be developed or have never been developed (link).
Non-chemistry/analytical (chemistry) science experts have difficulty understanding this issue, let alone solving it. Please seek help from the people in the areas of testing (analytical or chemical) science.
The issue is not as complicated as presumed or presented. Viruses and pandemics will disappear quickly.
(Video) COVID-19: An online discussion with Dr. Andrew Kaufman (USA) and Kamala Taris (Czech Republic)
(Video) COVID-19: An online discussion with Dr. Andrew Kaufman (USA) and Kamala Taris (Czech Republic)
(Today) I had an opportunity to discuss PCR testing related to its deficiency/invalidity as a test. Also discussed was the lack of scientific merit of the virus’s isolation, vaccine development, etc. I hope that you will find the discussion informative and useful.
Click on the picture to play the video
Please donate for my blog (by clicking the button below). Thanks.
Contacts and further information:
Dr. Andrew Kaufman: https://andrewkaufmanmd.com
Kamala Taris:
Resetheus Association (Return to Essential Scientific Ethos and Transparency, link, link)
Expert Statement on SARS-CoV-2 Testing (in Czech. link)
SOVI (in Czech, link)
COVID-19: Question more!
Look what I found on Amazon!
Zoom Meeting with a UK Panel
Zoom Meeting with a UK Panel
A couple of days ago, I participated in a Zoom meeting with a UK panel (physicians and others) on the situation (link). The meeting was very long; however, if interested, consider watching at 12:47 and 58:15 (in the latter part, I did not participate as I was not aware that the meeting continued after the disconnect).
It indeed appears that confusion is there, or created, to gain some advantages. Also, it remains questionable practice, at least to me, to treat people, the healthy ones in particular, with a new treatment, which is still in a developmental phase.
As noted here (link), I believe that we may be seriously in a misdiagnosis and mistreatment situation. This situation should not continue.
(Link to the first part of meeting/discussion)
When “isolation of a virus” is not the isolation
When “isolation of a virus” is not the isolation
As a part of a LinkedIn discussion (link), I provided the following (below) response to a query. The visitors of this blog may find my response informative and useful as well.
_________________________________________________________________________________________
Steve:
Thanks for your kind words about my article and also for asking the questions.
First, to be clear that I am not a microbiologist or virologist. I am a chemist and have worked in the pharmaceuticals area for 30+ years (as a scientist with Health Canada). I gained significant experience and expertise in critically evaluating pharmaceutical products.
Regarding the claim of virus isolation, I am saying that the experiments microbiologists/virologists perform and describe, such as virus characterization, identification, belongs to the chemistry discipline. However, the chemistry work has not been conducted accurately; hence claims made are incorrect.
There are many ways to describe and explain the inaccuracies. One of which is that of isolation of a substance, in this case, a virus. If one needs to isolate a virus, one must go through multiple steps, such as extraction, purification, identification, and structure determination, resulting in a pure sample of the virus. Nothing of this sort has been done for the virus isolation, in particular, SARS-CoV-2. Therefore, it cannot be said that the virus has been isolated and identified, or even it exists.
On the other hand, microbiologists and virologists (among others) work with a modified definition and description of the term isolation, for their purpose, as taking a swab sample (i.e., separating virus from the host). That is not a good scientific practice. Therefore, in reality, a virus never gets isolated in its true or pure form.
On the other hand, to appear scientific, the DNA/RNA sequencing is considered as a claim of “identifying” the virus in a swab sample without truly “isolating” it (we chemists call it a clean-up step). Some chemical steps using enzymes (commonly known as polymerization, again a chemistry step) are conducted, followed by taking some pictures of the soup with an electron microscope. Observing spherical bodies with spikes are considered to reflect the existence of coronavirus.
From this soup (note everything is from the soup, nothing from pure virus), DNA or RNA or its fragments are extracted to establish their sequences. There is no evidence that this DNA or RNA is from anything specific, including the virus – it is an assumption. Based on computer analysis and comparison with previously obtained “reference” sequence (usually obtained from WHO depository), which is also “isolated” in a similar manner (without isolating the virus), virus existence is established. If the sequence did not match the “reference” sequence (not a virus), it would become a new virus or new strain.
The publications, links you provided all follow the same or similar protocol as I summarized above. I have critically reviewed two such publications, one from Australia (the one you noted in your post as well), the other from the USA (CDC), where I explained: “science” behind “isolation” of the virus. Links are provided (link1, link2) please have a look.
I have been arguing for some time that nowhere I can find an isolated virus, so why people keep claiming isolated virus. My recent discussion with a microbiologist made it clear that the virus has never been isolated but misrepresented by incorrect definition of the word “isolation.” That makes it clear why I could never find the sample and specimen of the pure virus because it does not exist. One may imagine my shock after hearing this – so I wrote the article.
I hope I answered your query adequately. Otherwise, let me know. I will explain it further. I like to make another point, without going into technical details, the sequencing (chemistry) part is pretty iffy. It is well known to the people in the area that sequencing steps can produce highly unpredictable results. The PCR test, which in reality is based on sequencing, suffers this weakness. Hence one sees so many false positive or negative outcomes that make the lack of “virus existence” claim even stronger. (edited)
COVID-19: The virus does not exist – it is confirmed!
COVID-19: The virus does not exist – it is confirmed!
During a discussion on LinkedIn (link) with one of the microbiologists, I came to know how they described virus isolation, which is as follows:
“A virus isolate is a virus isolated from an infected host. The process is called “isolation,” which separates viruses from the hosts.”
It means that for microbiologists and virologists, taking a swab sample, which separates virus from the host, is considered as “virus isolation.” This interpretation does not reflect the correct meaning and understanding of the subject of isolation. But, they imply and promote the true meaning of the process of isolation, i.e., to obtain something by extraction, purification, and identification, reflected by well-known pretty pictures of the DNA/RNA, proteins, and viruses such as a spherical body with spikes (aka coronavirus). Continue here.
People are in great danger and need help!
People are in great danger and need help!
I am probably the first to describe the virus’s non-existence by critically reviewing the articles published from Australian and the USA (CDC) institutions. As expected, I got all the humiliating and insulting responses, basically stating that I am an idiot and have no understanding of the subject, making false claims, and spreading misinformation. I patiently listened, and politely responded to these responses.
I am starting to see people disappearing from such discussions now. They are probably embarrassed by their position and views. They were wrong, realizing that there is no virus (SARS-CoV-2) and, by extension, no COVID-19 pandemic.
I am correct, and they are wrong because the problem they are dealing with is not of medicine, virology, or epidemiology but chemistry. Virus isolation, characterization, testing, polymerization, and structure determination are part of chemical sciences or chemistry, and they have no training, even the basic one, in this subject. Given the opportunity, anyone with some reasonable chemistry expertise can find out about their false science and claims.
Their trickery could not be exposed earlier because; it was always kept within the bounds of their discipline, i.e., protection was provided by one another within the “group” – the so-called peer-reviewed process.
However, now inquiries are coming from outside. For example, show us that the (PCR) test is valid (a chemistry topic, they cannot answer withTHEIR “science”); show us the virus (chemistry topic, they cannot answer it with THEIR “science”).
They have no valid answers, except to follow the teachings or propaganda of their fad leaders. People are misdiagnosed with the virus, and its disease, which does not exist. Someone has to take responsibility for this mishap. They are doomed or soon to be.
I worry about the vaccination of the public imposed based on their false science and claims. The vaccine itself is a chemical compound, claimed to produce another chemical compound in the body that will then kill the virus (an unidentified virus, if and when it comes).
The vaccination of masses with chemicals to protect them from a virus, and its disease, which does not exist, is cruelty. At this stage, it is a pure and simple injection of potent chemicals, not adequately tested, into the human body. It is simply inhumane! I hope that somehow this process would be stopped before it is too late. Can someone, please God, help us in this regard? We, the people, are in great danger and need help!
The FDA Committee’s Review of Pfizer-BioNTech COVID-19 Vaccine: Unscientific, False and Deceitful
The FDA Committee’s Review of Pfizer-BioNTech COVID-19 Vaccine: Unscientific, False and Deceitful
On December 10th, 2020, the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC or the Committee) hold a meeting to evaluate the Pfizer-BioNTech vaccine for the COVID-19.
This article provides a critical review of the underlying scientific aspect of the evaluation process and its outcome. The Committee’s judgment is considered biased, lacking scientific vigor – including for chemistry and manufacturing, and misleading for describing efficacy and safety assessment for the vaccine use. Continue here
SARS-CoV-2 (virus) and COVID-19 (disease) – Questionable Narrative!
SARS-CoV-2 (virus) and COVID-19 (disease) – Questionable Narrative!
The virus (SARS-CoV-2) has never been isolated, positively identified, or physically characterized. COVID-19 does not have defined, specific or measurable symptoms and has never been linked to SARS-CoV-2 experimentally (as it has not been isolated and identified). A strong possibility exists that COVID-19 could be an incorrectly diagnosed disease – may be an imaginary one. Therefore, scientifically the implementation of vaccination should be considered an incorrect or false treatment.
Discussion on the SARS-CoV-2 virus and its associated illness using scientifically invalid PCR test
Discussion on the SARS-CoV-2 virus and its associated illness using scientifically invalid PCR test
As a part of a discussion on LinkedIn (link), I posted a response highlighting grievous misunderstanding and misinformation concerning the PCR test’s relevancy and scientific validity. My posted commentary appears missing from the forum, along with the concurrent disappearance of Dr. Daniel Goldstein’s numerous comments. I believe my analysis is valid and would be helpful to others for future discussions on the topic. The visitors of this blog may also find my post (below) useful as well.
Response to: Daniel Goldstein, MD (Professor & Vice Chairman, Department of Cardiothoracic Surgery Montefiore Health System), link
Please be nice and civil. If you do not respect yourself, consider respecting your profession and the organization you may be representing.
Thanks for pointing out the typo on my blog. I will go through it and will correct it if required. You may also suggest where you found the error specifically, which would be helpful. On the other hand, under discussion is the validation of the PCR test, which is not affected if my blog has some minor typos.
Regarding the post “blocked from LinkedIn” on my blog, I do not consider it a bad thing but a good. Otherwise, I would not have put it there. As I have pointed out before, my views are not in line with current popular belief but are based on independent thinking, expertise, and analytical science experience. This aspect is missing from the present projected and promoted opinions.
Eléna Diakaki is following my work for some time. Based on my description of the issues (virus, testing, illness, pandemic, etc.), as I usually describe in a simple and mostly non-technical language, she, like many others, agrees with me, at least understands the issue well, and are questioning the popular view. If you do not agree with my perspective, or have a different opinion, share your thoughts with supporting science and data in a simple language so that people can understand. There is no need for jumping up and down and shouting. You do not realize that your responses clearly show that you do not have anything valid in support of your argument, except telling just follow others like you are following.
Everyone is working to improve the health and happiness of people. You are not the sole custodian of this aspect. From my perspective, as it is a well-known and well-established fact that PCR tests are flawed and bogus (you may believe or say whatever you like), so the testing must be stopped immediately. The pandemic will disappear in a blink of an eye as well. People are getting labeled (diagnosed) incorrectly with this flawed test. Once the testing stops, patients come to the hospital, assess them accordingly and appropriately, having an open mind.
I hope you will consider the issue under discussion calmly and explain why you believe the PCR test a valid test. BTW, as I mentioned before, you might have difficulty in understanding the question, so please seek some help from someone knowledgeable in the testing area and validation of tests.
In the end, I would say that I may get blocked from LI again. People are and will be doing badmouthing. If that happens, please reach me through my blog (www.drug-dissolution-testing.com). I will be happy to provide my opinion to you or anyone else to the best of my abilities.
Good luck
Understanding clinical trials and their outcomes – fake science at its best!
Understanding clinical trials and their outcomes – fake science at its best!
Clinical trials are specific type of tests in which products or treatments are tested using human subjects. Such trials should be conducted under the management of analytical science or laboratory with scientifically valid protocols. Unfortunately, however, at present trials are conducted in non-analytical science facilities and supervision often with invalid study protocols and interpretations. Approved products based on such trials would provide false assurance of safety, efficacy and quality of the tested products. Patients and public should be aware of current flawed scientific practices in this regard. On the other hand, if such evaluations are conducted with appropriate and scientifically valid and proven approaches, one could not only avoid false mishaps but also develop treatments and cure far more expeditiously and cost effectively. click here or here to read complete article
Ending the COVID-19 pandemic – simple and scientifically valid approach
Ending the COVID-19 pandemic – simple and scientifically valid approach
COVID-19 pandemic has been established based on testing of patients and the public in general. It is, therefore, a legitimate question to ask that the authorities and/or experts to provide the details and validation data of the tests they have used to establish the presence of actual virus (SARS-CoV2) and/or COVID-19. On the other hand, it is also a common knowledge that a scientifically valid COVID-19 and/or virus test is neither available nor can be developed at present. It is, therefore, becomes a fact that presence of the virus and its disease, and by extension pandemic, cannot be establish and confirmed. In short, the disease and pandemic does not exist and authorities consider declaring ending of the COVID-19 pandemic claims. (1, 2)
COVID-19: Vaccine ‘Not Possible’ For A Virus Not Yet Quantifiable
Is ‘COVID Vaccine’ A Copycat Of Theranos Blood Test Fraud?
COVID-19 (pandemic) testing vs Theranos’ blood testing – similarity of underlying false/fraudulent claims
COVID-19 (pandemic) testing vs Theranos’ blood testing – similarity of underlying false/fraudulent claims
If people are not aware of the Theranos’ blood testing scandal, a quick Google search will reveal the details. The following is from Wikipedia (https://en.wikipedia.org/wiki/Theranos).
“Theranos was a privately held health technology corporation. It was initially touted as a breakthrough technology company, with claims of having devised blood tests that required only very small amounts of blood and could be performed very rapidly using small automated devices the company had developed. However, the claims later proved to be false.”
“After all efforts to find a buyer went nowhere, what remained of the company dissolved on September 4, 2018.”
The underlying cause of Theranos’ demise, a reportedly 10 billion dollar valued company, was promoting and selling non-validated test or testing.
Similarly, if one would request validation data for COVID-19 testing (presence/absence of virus and by extension its disease, not of some non-specific proteins) in particular from authorities/experts, it will immediately become clear that claims of testing/pandemic are false. However, in this case authorities are making and accepting false claims for the testing and avoiding the Theranos like demise which would be unavoidable for long. Request the authorities to provide validation details of the available COVID-19 tests? The pandemic will disappear very quickly as did the Theranos.
For requesting validation protocol/details please follow the links (1, 2).
Pandemic: why and how? Certainly testing is irrelevant!
Pandemic: why and how? Certainly testing is irrelevant!
Considering the statements below, from a randomly selected fact sheet FDA approved under Emergency Use Authorization or EUA, the current antibody testing lacks scientific validity. This is really sad that such tests [kits] are being promoted or used to establish COVID-19 [1]. As noted below the test monitors protein levels commonly known as [IgM, IgA and IgG] not specifically COVID-19. Logically data obtained from such tests should be avoided in making predictions or projections about the infection and its spread. It certainly is a false science.
1. “A positive result with VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack test may not mean that an individual’s current symptoms are due to COVID-19 infection.”
2. “However, a negative result does not rule out COVID-19.”
3. “The absolute sensitivity of the VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack test is unknown.”
4. FDA statement [2] “This limits the test’s effectiveness for diagnosing COVID-19 and why it should not be used as the sole basis to diagnose COVID-19.”
In addition, if vaccines would be developed based on such antibody tests, which does not appear to be sufficiently validated as noted above, then how reliable and valid vaccines would be? Please be cautious with claims in this regard.
Will therapeutics/vaccines be developed for COVID-19? Of course – but only fake ones!
Will therapeutics/vaccines be developed for COVID-19? Of course – but only fake ones!
A logical and scientific requirement for such (i.e. development of therapeutic/vaccine) is the availability of a VALID analytical/clinical test to establish the presence/absence of Coronavirus (SARS-CoV-2). However, unfortunately, at present, there is no such VALID method exists. Therefore, scientifically speaking, it is impossible to establish the presence/absence of COVID-19 in humans with sufficient accuracy/specificity and by extension development of its treatment/cure either by pharmaceuticals or vaccine.
Media and bureaucracy can produce anything (virus as well as its treatment) out of thin air that is a different story. If people like to believe in such stories then that is their problem.
Every test and/or testers come with a calibration/validation certificate. Similarly, the COVID-19 test has to have calibration/validation based on parameters i.e. method validation data such as (1) accuracy; (2) precision; (3) specificity; (4) references used to validate the method. It is a standard and normal practice/requirement for any analytical/clinical test. Unfortunately, this is missing, hence all the claims made about the presence/absence of COVID-19, and/or its treatment/cure, MUST be considered unscientific or false.
Further details (link)
Do FDA and USP lie? Of course, all the time!
Do FDA and USP lie? Of course, all the time!
For example:
FDA claims that it establishes and monitors quality of pharmaceutical products such as tablet and capsule. A lie – FDA neither defines quality of the products nor its measurable parameter hence it does not, or cannot, determine quality of the products.
FDA claims that it establishes safety and efficacy (as well as quality) of pharmaceutical products using valid clinical testing (e.g. bioequivalence assessment) and in vitro (drug dissolution) testing using USP apparatuses. A lie – these tests, along with associated testers, have never been validated for the intended purpose. In fact, these tests have been shown to be scientifically invalid and irrelevant for their intended purpose.
USP claims that it provides reference standards for establishing quality of the pharmaceutical products such as tablets and capsules. A lie – USP never provides reference standards for any product. It provides powder or liquid samples of pure chemical compounds, not the products which patients use, however falsely promotes as reference standards of medicines.
USP claims that it provides a valid analytical test for the assessment drug release characteristics of the products for establishing and monitoring quality of the products. A lie – the test has never been validated for the intended purpose. The test cannot determine drug dissolution/release characteristics of any product. It has been shown experimentally that the test provides irrelevant and highly unpredictable results/data with no relevance to product quality.
For more examples please visit here. Manufacturers and patients should be cautious in accepting such claims from FDA and USP as well as other national and international authorities which often follow FDA/USP claims and guidances.
Please consider accepting the Citizen Petition (under review with FDA for more than a year and a half, link) for addressing the underlying lies concerning products development, manufacturing and their regulatory approval.