Monthly Archives: April 2011
Dissolution-Absorption Link
Drug Dissolution Testing Mosaic
Dissolution Test For Nicotine Polacrilex Lozenges
In response to a query, I have provided the following opinion on the above mentioned topic. I thought this topic may have general interest, thus posting it on the blog as well. Comments/critiques are always appreciated.
_______________________________________________________________________________________________
Thanks for your interest in my work and the website.
Concerning your question regarding dissolution test for Nicotine Polacrilex Lozenges, first of all I must say that I personally have not worked with such products. Therefore, consider my advice as a theory (quick thoughts) which may be workable with some experimental work.
As you stated that the release of drug (nicotine) from the lozenges occurs in 30 minutes in humans, however, in vitro test requires 8 hours for the release. Obviously somewhere there is a mismatch between in vitro and in vivo environments. Considering that there is a higher pH (6.2 to 7.4) in the oral cavity than in the intestinal tract, the suggested use of a higher pH of 7.4 for dissolution appears appropriate. My preference would be around 6.0
To me, however, there appear to be two issues here. First, there may be a lack of needed stirring and mixing within the dissolution vessel. I am of the view that the use of the basket/paddle apparatus would be the least efficient choice here. Nicotine Polacrilex is an ion-exchange complex, thus may require some modest “shake” to pull the drug out of the polymer. Thus, it may require a different mechanism of stirring, may be a crescent-shape spindle as I have proposed, or something of that nature which should be good and efficient in moving the medium from the surface and in providing mobility to the lozenges.
The second aspect is related to chemistry. The complex between nicotine and Polacrilex is ionic. Thus, you may need some form of competitive cation in the medium, which would facilitate the pull-out or replacement of nicotine from the ion-exchange. Otherwise ion-exchange may keep the nicotine from releasing, which would result in an extra ordinary delay in release (8h?). It is to be noted that the working at pH 7.4 (dissolution) is not very favourable to this dissociation, as the pKa of nicotine is 8.5. Having said that please keep in mind that if you are going to try a competitive cation, this has to be a bio-relevant one, otherwise, your dissolution test will lose bio-relevancy. Perhaps, CTAB may be tried, a commonly suggested solublizer (surfactant) for dissolution testing.
Hope this will help.
Operating principle of a dissolution tester (Paddle/Basket)
The main operating principle of a paddle/basket (or vessel-based) apparatus is to provide a precise and controlled stirring and mixing mechanism at 37 C. In reality, from the operational aspect a beaker with a magnetic stirring bar may be considered equivalent to a dissolution tester if the rpm of the stirrer is precisely controlled and beaker content can be maintained at 37C. Therefore, it is important to note that operation of a dissolution tester should be as simple as any other stirring device.
 To achieve time savings and consistency in results, the current dissolution apparatuses come in units of 6 or more stirrers with appropriate mechanical and electronic controls. However, operating principle remains the same whether the apparatus is based on a single or multiple stirring units.
To achieve time savings and consistency in results, the current dissolution apparatuses come in units of 6 or more stirrers with appropriate mechanical and electronic controls. However, operating principle remains the same whether the apparatus is based on a single or multiple stirring units.
Generally, stirrers (or stirring devices) come with their limitations. For example, a magnetic bar may not be suitable for mixing viscous or solid materials because bars lack sufficient torque and the stirred contents are not easily move able. Similarly, basket/paddle stirrers cannot be used as mixers for solid materials (tablets/capsules) as these settle at the bottom of the vessels where flow of the liquid (medium) is low to negligible. A representation of lack of interaction between solid/liquid with such apparatuses is shown in the figure, which is known as “cone formation”. Therefore, by its nature basket/paddle stirrers (or apparatuses) cannot be used where thorough mixing (interaction) of solid/medium is required, such as what occurs in the GI tract.
Furthermore, the random settling positions of a product (tablet/capsule and their particles) at the bottom of a vessel add variability to this mixing, thus dissolution results would be erratic and unpredictable.
To sum up, the operating principle of the basket/paddle apparatuses is based on a simple stirring device. However, operational limitations of such stirrers are such that they cannot provide efficient product/medium interaction or mixing, thus would provide erratic (highly variable) and unpredictable results.
Dissolution method precision – The way I see it
In a recent article titled “Evaluation of In-Situ Fiber Optics Dissolution Method for Compound A Extended Release Tablets” published in the March/April issue of American Pharmaceutical Reviews (Link), the authors described the usefulness of the technique for dissolution testing. The usefulness is described based on method validation parameters such as specificity, linearity, accuracy/recovery, precision, robustness etc.
To me the precision is the most important parameter as almost all the other parameters depend on it, i.e., if the precision is not acceptable then the other method validation parameters will also reflect poorly, thus the method itself.
It is very well accepted and common practice to describe the precision of a method based on Relative Standard Deviation (RSD) or Coefficient of Variations (CV) in percentages. However, in the article (method and intermediate precisions, Tables 5 and 6) authors reported no %RSD (CV%) values. Therefore, the question is how is the precision of the method established? Moreover, why would the authors omit the precision (%RSD) values? Continue reading
Obtaining bio- (physiologically) relevant dissolution results
It is often highly desirable to obtain bio-relevant results as such results increase the confidence and usefulness of the testing. In fact, one should always focus on achieving bio-relevant results as non bio-relevant results are of limited use.
To obtain bio-relevant results one should try to evaluate products using experimental conditions as close as possible to the conditions one would expect during the physiological testing and/or the use of the product.
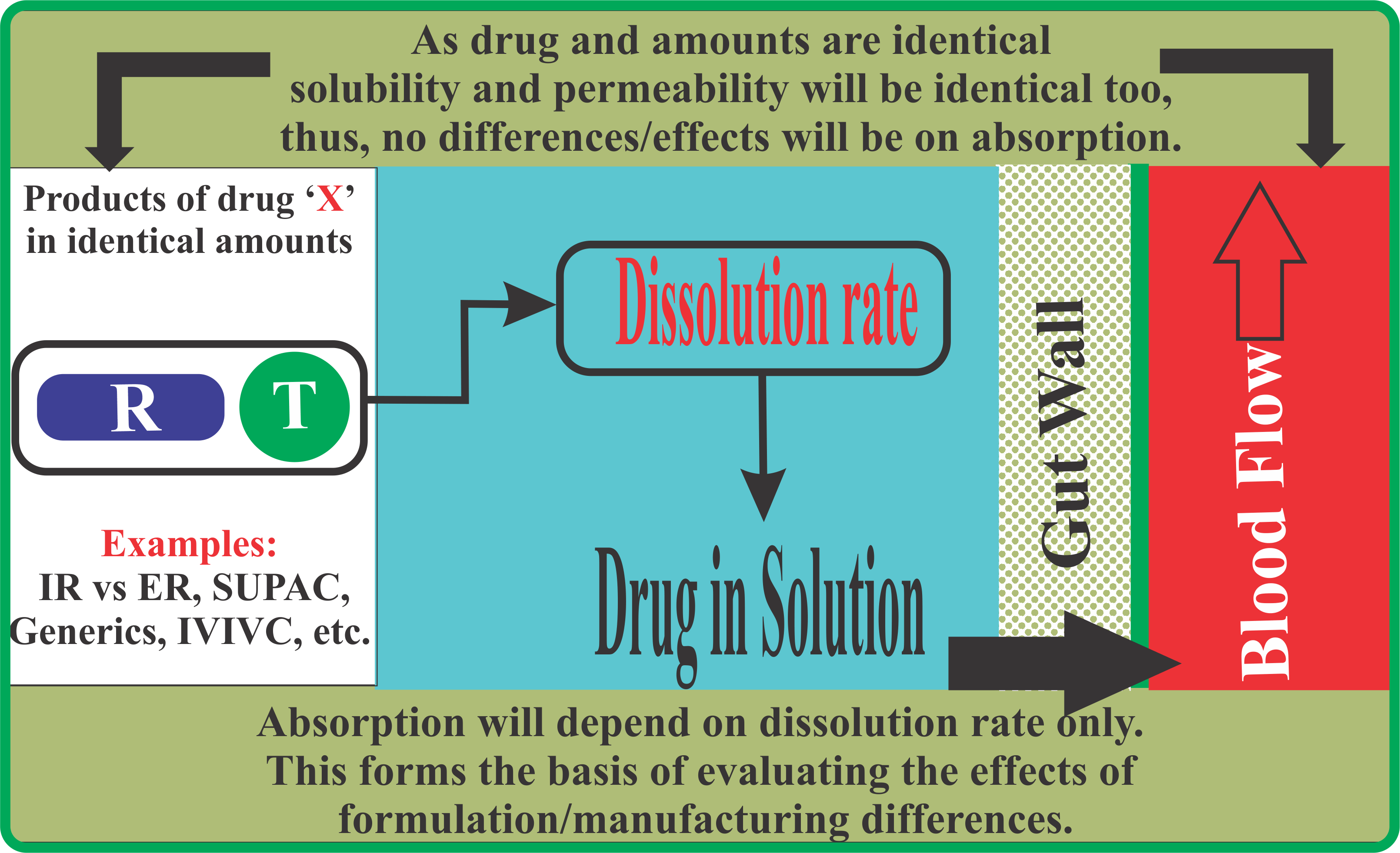
For the evaluation of in vivo drug dissolution, as conducted based on the bioavailability/bioequivalence studies, it is a common regulatory requirement that products are tested using a standard and common protocol. For example, for the evaluation of IR vs ER products, and any release type in between, the study protocol (physiological environment) remains the same. Therefore, if one wishes to achieve bio-relevant results then one has to conduct in vitro testing using a common set of experimental conditions and these should be product independent as well.
Conducting dissolution studies using product dependent experimental conditions violates this principle, thus should not be considered bio-relevant. To obtain bio-relevant results, testing should be done using a common set of experimental conditions which should also be product independent.
Info
Today, I have added a list of my selected publications on the subject, under the section (sub-heading) of “Useful Lists”. Hope you will find the articles useful.