Monthly Archives: June 2011
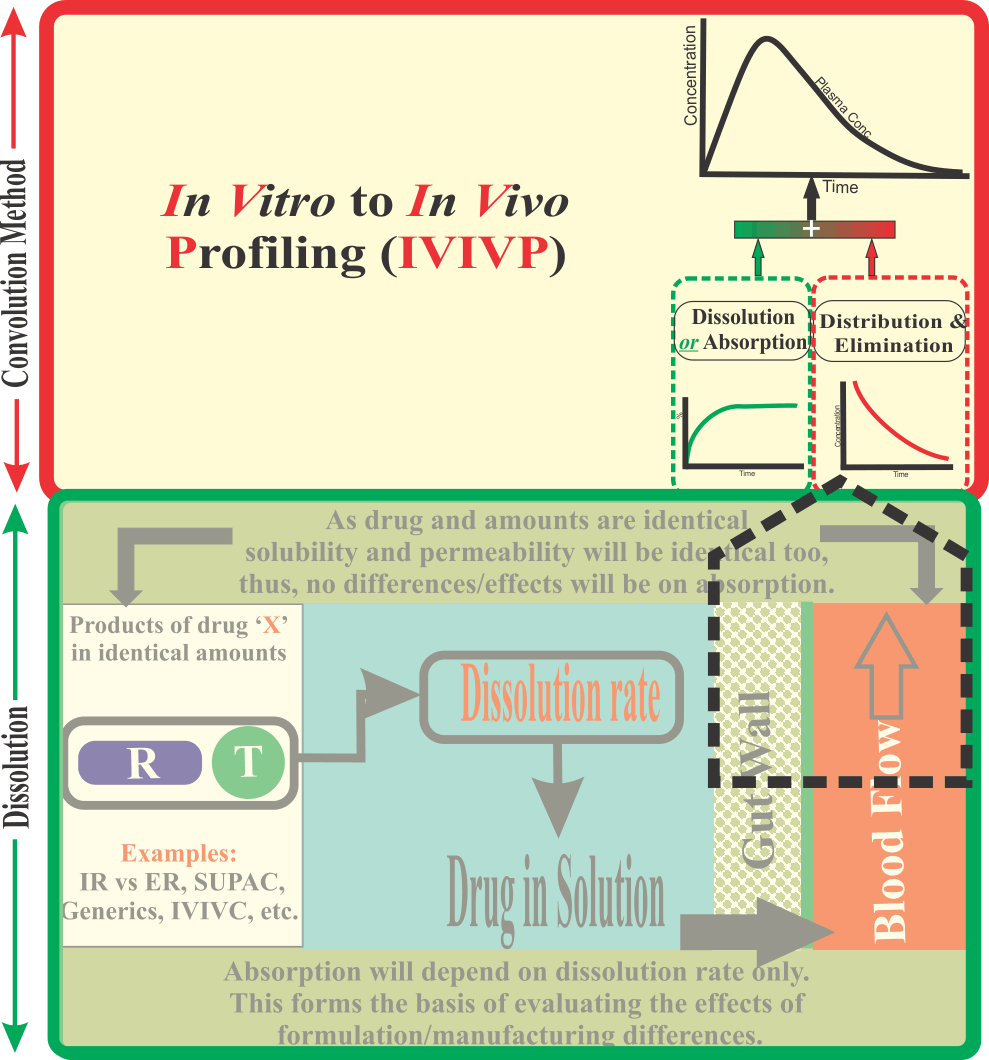
In Vitro-to-In Vivo Profiling (IVIVP)
Has BCS (Biopharmaceutics Classification System) been a Futile Exercise?
It appears so.
BCS is an approach which has been propagated to reduce the burden of pharmaceutical product evaluations and their regulatory approval using drug dissolution testing instead of in vivo (bioavailability/bioequivalence) testing.
It should be noted that in reality the BCS concept was introduced to facilitate success in establishing IVIVC, which in general had shown poor, or no, success.
The underlying principle of BCS is that if drugs are classified into classes based on their aqueous solubilities and absorptions or permeabilities through the GI tract, then establishing IVIVC may be possible. It is, therefore, important to note that the concept of BCS was introduced to increase the chances of IVIVC success.
For BCS, drugs are divided into four classes having the characteristics of: (I) High solubility and high permeability; (II) Low solubility and high permeability; (III) High solubility and low permeability; (IV) Low solubility and low permeability. As per BCS approach, for drugs which would fall in the low permeability category i.e. classes III and IV, it would be unlikely to achieve successful IVIVC as drug dissolution testing does not relate to permeability. Thus, success of IVIVC may not be possible for the drugs of these classes. The drugs in class I are also considered as poor candidates for a successful IVIVC as these drugs would often dissolve so fast that they may overwhelm the absorption system. Therefore, the drugs in the class II (low solubility and high permeability) and drugs of class I, where release would be manipulated so that drugs appear as slow dissolving (e.g. extended-released type) would have the potential to achieve successful IVIVC. It is important to note that in principle BCS would support potential success of IVIVC with only class II type drugs. However, reported success of IVIVC for drugs in class II has also been limited, (perhaps because of mismatch of in vitro and in vivo environments, see link) and one should be cautious when describing the BCS as a success or useful practice. Thus, the extension of BCS in developing IVIVC and its usefulness in regulatory environment should be considered with care.
On the other hand, there are Guidances available which suggests that the use of the BCS concept for bio-waivers, meaning products may be evaluated using in vitro drug dissolution tests only without in vivo testing. First of all, these Guidances are applicable for products of drugs in class I only, where generally it is recognised that the achieving IVIVC would be highly unlikely based on the BCS concept as explained above. Moreover, there are other conditions as well which these drugs and their product must also meet. For example, products must be of immediate release type and the products must also release the drug very quickly, generally in less than 30 minutes. In essence, the products for which bio-waiver may be considered should be such that they should have drugs which would be released and dissolve quickly. The assumption here is that the human body will consider these products equivalent to solution products. It is obvious from this discussion that the BCS which intended for developing IVIVC plays a limited role in describing bio-waiver criteria for this particular class of drug where no IVIVC is expected.
It may, therefore, be concluded that BCS had been of limited use in facilitating IVIVC, application of dissolution testing in lieu of in vivo testing and/or reducing regulatory burden.
Dissolution method development – a practice which causes confusion and hinders in product evaluation
In my opinion, current practices of method development have not only caused the biggest confusion in the industry but also hindered in an appropriate evaluation of drug products using dissolution (release) testing itself.
Current practices of method development suggest that an analyst is to “fish” (seek) experimental conditions, such as choice of an apparatus (paddle/basket), rpm, buffer, pH to establish UNKNOWN dissolution characteristics of a product. Normally, it is suggested that an analyst should have a number of formulations with presumed dissolution characteristics and the analyst should seek experimental conditions which would reflect his/her “presumed” product characteristics. Such practices are often also referred to as developing “discriminating” and/or “lot-to-lot consistency check” methods.
Similarly, the above mentioned practice of “fishing” (seeking) experimental conditions will be considered as developing a bio-relevant method if the analyst tries to match dissolution results with in vivo results (bioavailability/bioequivalence).
Therefore, in practice, an analyst would never know the true product dissolution characteristics but would select experimental conditions which would fit his/her expectations. It is therefore, critical to understand that as it stands now, an analyst or a product developer will never know the true dissolution characteristics of its product. Each and every analyst/product developer is occupied with “developing” dissolution methods which in reality should not be their assignment. Their objective is to develop a product based on its dissolution characteristics, using a standardized and well accepted dissolution method. This is similar to an analogy in which each and every laboratory would be busy in developing their own thermometers and weighing balances to be used for monitoring temperatures and weighing substances, respectively.
It is therefore, essential to note that the pharmaceutical industry requires a standard dissolution tester along with its associated experimental conditions capable of providing dissolution characteristics of pharmaceutical products. The crescent-shape spindle has been developed with these thoughts in mind, which appears to offer a powerful solution to the current confusion and avoids the unnecessary practices of method development.
Concept of “quality” in drug dissolution testing
In the drug dissolution testing area, a reference to “quality” of a product (table/capsule) is frequently made. For example, a drug dissolution test may be considered as a quality assurance and/or control test. More recently use of the term has been extended to the concept and practice of “quality by design” or QbD. It is obvious that to achieve or monitor “quality”, one needs to define it as an achievable objective or goal.
In general terms, “quality” is a subjective term for which each person or sector has its own definition. For example, certain variations in shape, size or color of a tablet may be an indication of poor quality of product for some but for others it may be normal and expected. In the literature, commonly “quality” refers to “fitness for use” or “conformance to requirements” according to Joseph Juran (considered father of the QbD concept) and Philip Crosby, respectively. However, in technical usage such as for the assessment of pharmaceutical products, these definitions of “quality” may be translated into a property of a product which fulfils stated or implied needs by establishing it as “fit for use”. The terminology of “to fulfil stated or implied needs” appears to be the most critical in this regard, i.e, one has to establish the need before even defining the quality.
Therefore, before using dissolution testing for “quality” assessment purposes (or its control/assurance), one first has to describe what is the stated or implied need here. Until and unless one cannot define or establish a need or use (for the testing) one cannot establish the quality. What is the need or use of a drug dissolution test? Conducting a dissolution test is not a need by itself. However, it is the ability (test) to fulfil a need of determining drug dissolution in human GI tract from pharmaceutical products. The need is determining of drug dissolution in human GI tract. This particular needed characteristic reflects upon the “quality” of the product. How it is to be determined and controlled or assured comes later, which is the “fitness for use” characteristics. In this regard, both, i.e., the use of bioavailability/bioequivalence (BA/BE) testing/studies and drug dissolution testing are two different types of tests to fulfill the same need and establishment of “fitness for use” criteria. One reflects the in vivo evaluation while the other in vitro but they both fulfil exactly the same need. One may argue about the differences in the strength of these two types of tests, but one has to recognize that they both fulfill the same need. Once the need is established, then one has to establish tolerance (specifications) for “fitness to use” around the needed characteristics, so that this need may be fulfill consistently. For further discussion on the evaluation of drug release (dissolution) both in vitro and in vivo, please see the post.
In short, therefore, “quality” here is referred to as fulfilling a need of determining the drug release (dissolution) from a product in the gastrointestinal (GI) tract, which is quantitatively measured to establish the characteristic of “fitness for use” by a drug dissolution test.
Apparatus Calibration or Performance Verification: Misleading Conclusions and False Comfort
In a recent issue of Dissolution Technologies, in an article titled “Overview of Dissolution Instrument Qualification, Including Common Pitfalls” the authors started the article with a statement (or quote) of the claim that “For almost fifty years, the pharmaceutical community has been relying on dissolution data as an indication of drug product performance. Effective qualification of the dissolution apparatus is critical to the value and integrity of these data”.
The above mentioned statements can be misleading and may provide false and erroneous comfort for a drug product performance using instrument qualifications (IQ) as its basis, as explained below: Continue reading
In Vivo vs In Vitro Bioequivalence
In vivo bioequivalence, or simply bioequivalence, is commonly referred to as an evaluation study conducted to establish equality of mostly two oral products such as tablet or capsule. Equality of two products (test vs reference) is established by comparing their blood drug concentration-time (C-t) profiles. The reason for selecting C-t profiles for such comparison is that as therapeutic effects depend on drug concentrations in blood i.e., if two or more products provide similar C-t profiles then they will provide similar therapeutic effects as well, thus they will be considered therapeutically bioequivalent, or simply bioequivalent. [Continue …]
Lack of faith in obtaining physiologically relevant dissolution results. There is a reason for it.
It is often stated that in vitro drug dissolution testing may never be able to predict the physiological outcome as the environment, and processes within, may be too complex and variable to be adequately reproduced in vitro. This prevents to achieve adequate and bio-relevant dissolution results. This belief may not reflect the reality and appears to lack any experimental evidence.
The belief appears to be based on dissolution results obtained using mostly paddle and basket apparatuses. Interestingly, it has been shown repeatedly that these apparatuses poorly mimic physiological environment (e.g. see), which is required for dissolution testing. It is, therefore, should be expected that these apparatuses would not provide physiologically relevant results. In addition to a lack of physiological relevancy, it has further been shown that hydrodynamics within dissolution apparatuses (paddle/basket) is such that these apparatuses should provide highly variable and unpredictable results [link]. It is, therefore, safe to assume that it is not the difficulty and complexity of reproducing a physiological environment in vitro but the choice of dissolution apparatuses which appears to have caused the lack of success. Recent studies using the modified spindle (crescent-shaped) which addresses the artifacts of the paddle and basket apparatuses, appear to provide choice of physiologically relevant experiment conditions, thus providing improved and physiologically relevant results [Link]. The use of the crescent-shaped spindle provides a common and product independent testing environment one observes in vivo, where products are also evaluated under common and product independent environment. This is in contrast with the current practices of using paddle and basket apparatuses where practically each and every product is analyzed using its own method, a physiologically non-relevant condition.
It is, therefore, essential that for accurate dissolution results and their interpretation one should conduct drug dissolution tests using apparatuses which can simulate physiologically relevant experimental conditions. As the paddle and basket apparatuses provide a non-physiological testing environment, they will provide non-physiologically relevant results, thus the lack of the faith.
Drug dissolution testing for phase I clinical trials/studies
I received a query by email seeking my opinion concerning the topic mentioned above. The email is attached (link), without reference of the sender, to provide a background of my response. My opinion is as follows:
First, what is a phase I clinical study? In general, a phase I clinical study is a study in which a drug is to be evaluated in humans for the first time, following successful animals studies, to establish its safety and tolerability in different dosage strengths. In principle, at this stage, there would not be any data available on human pharmacokinetics (absorption, metabolism, elimination, volume of distribution etc), and this phase of the study is generally used for determining these parameters. If the drug is to be administered as a solid oral product such as a tablet/capsule or suspension, then such a product is to be “developed”. [link for full response]