Monthly Archives: September 2018
Dear regulatory authorities:
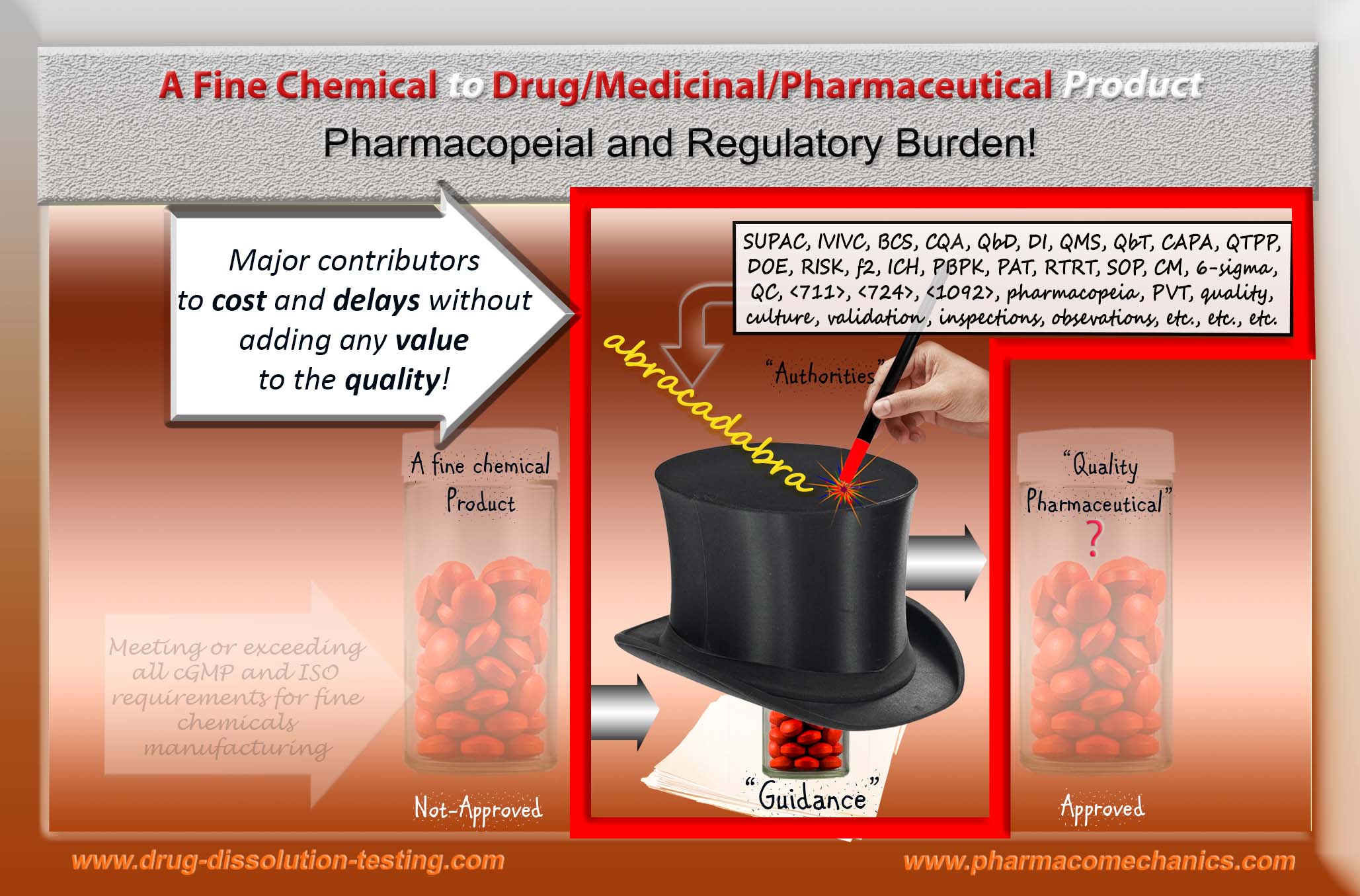
“Regulatory (pharmaceutical) science” – lacks logic as well as science!
One cannot establish quality of anything without knowing or defining it first. This is simple logic!
Regulatory authorities including pharmacopeias, however, have been trying to prove this logic wrong! That is, they have been making claims of establishing and monitoring quality of pharmaceutical products such as tablet and capsule – without knowing or defining it. Obviously, they will fail and have been failed!
Logically and/or scientifically, none of the products (approved or otherwise) available on the market can be considered of quality. Guidance and compliance-based system, along with the plant inspections, is a thick smoke screen hiding the reality and hindering the progress.
Please, define a quality product and set the standards and specifications accordingly so that appropriate quality products could be manufacture and monitored. For further detail, please see here.
Selecting medium for drug dissolution testing: Please pay attention to the principles of science and the laws of nature
Considering solubility characteristics of a drug in the stomach (i.e., pH range of 1 to 3) are pretty much useless from the perspective of absorption of a drug. Even if a drug gets dissolved in the stomach, it will be precipitated out in the intestine if it has lower solubility at a higher pH.
For absorption purposes a drug must dissolve, not necessarily completely but in some quantity, in the intestine where pH ranges from 4.5 to 7. Drugs get absorbed in steps in the intestine by continuous extraction process thus complete dissolution of drugs, at any given time (so called “sink condition”) is not necessary.
Moreover, dissolution characteristics are not usually determined for a drug – dissolution tests are conducted to evaluate products. The choice of medium is linked to the physiological environment of the GI tract not to the drugs or products. Please pay attention to the principles of science and the laws of nature. For further detail please follow the link (http://www.drug-dissolution-testing.com/?p=1749).