Monthly Archives: October 2016
Please consider the basis and do not make up the science!
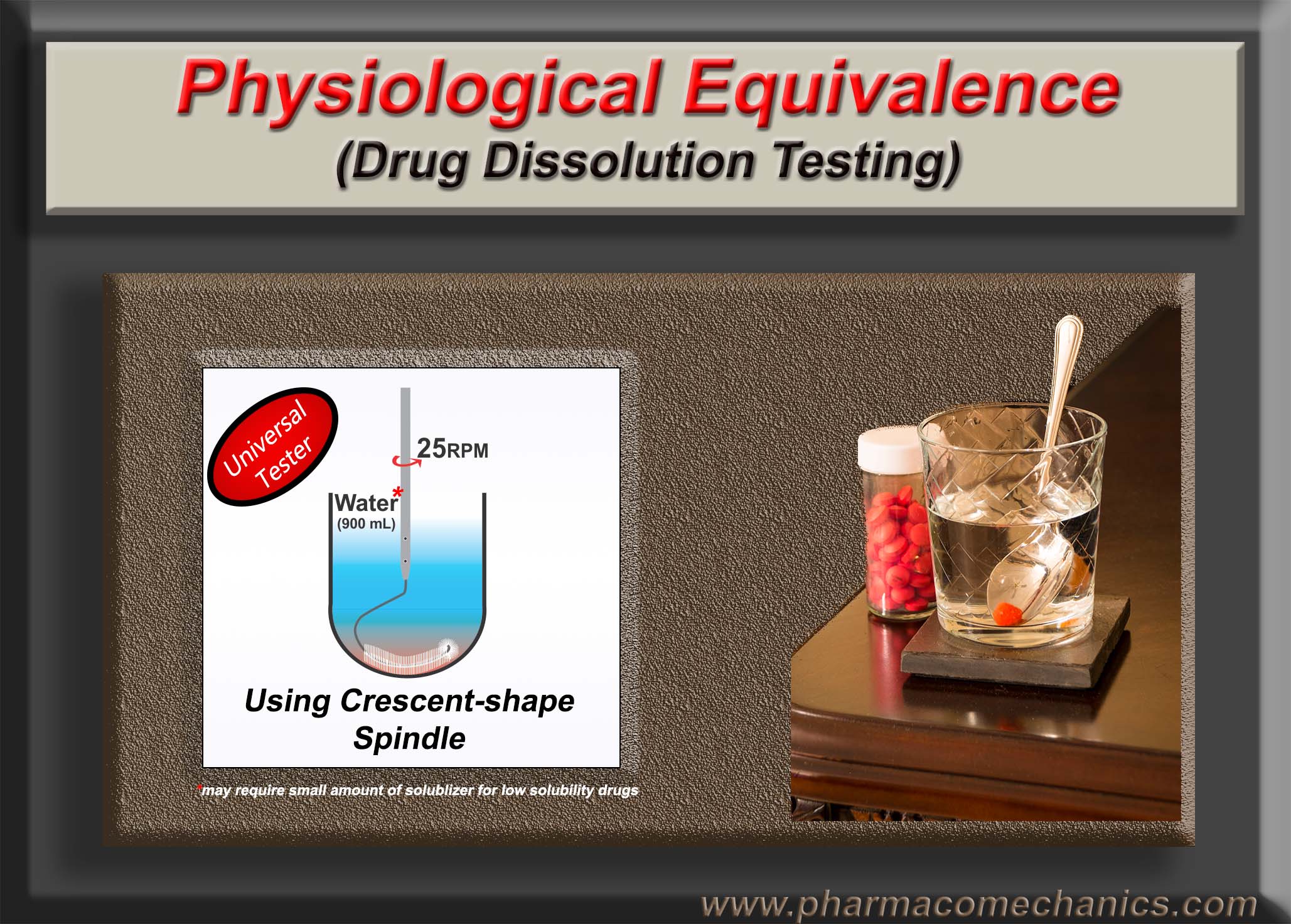
A simple and practical approach for establishing credibility and authenticity of the help and advice provided in the practice of drug dissolution testing
Drug dissolution testing is a well-established analytical technique or test, and is extensively employed for the development and assessment of pharmaceutical products in particular the tablet and capsule. In fact, it would not be an exaggeration in saying that it is the only test which is used for establishing the quality of products. However, it is also a fact that the tests and testers as recommended have never been qualified and validated for their intended purpose. Therefore, tests and testers cannot provide relevant and scientifically valid results regarding the quality of the tested products. This is simply common sense and scientific fact.
Some however, still promote the current practices, through trainings (conference, seminars, and write-ups) as scientific and useful which are not only causing delays and hindrance in addressing the issues at hand, but also create false hope for analysts/scientists that this technique may provide useful and relevant results. Sometimes this training and advice is disguised in different fanciful names or topics such as clinical or bio-relevant, IVIVC, bioavailability/bioequivalence assessment, but underneath it all are flawed dissolution results. Reality remains that it is almost impossible to obtain reproducible and relevant dissolution results for any product using the currently suggested dissolution testers.
A simple and practical approach one could use to assess the credibility or authenticity of promoted claims and expertise is to request if the person (trainer or vendor’s representative) is able to determine dissolution characteristics of a given blinded sample of a product containing a highly soluble drug using an independently developed and validated method (see below). If yes, then certainly it could be a good source for learning, otherwise, one should use caution.
Please use caution and pay attention (link)
Quality assessment and prevailing illusions!
The main promoted claim from regulatory authorities such as the US FDA, Health Canada and other national and international agencies concerning pharmaceutical products, such as tablet/capsule, is that they ensure that the public or patients receive quality medicinal or drug products. In this regard, it should go without saying that to establish quality of products in a scientific manner, one would require to define the quality of a product in an objective manner with a measurable metric. However, at present, regulatory authorities worldwide do not provide such a definition or metric (link). Therefore, it is not possible to establish and/or assess the quality of the manufactured products.
Current practices of proclaimed monitoring and/or establishing the quality of products are based on traditional views and opinions compiled as Guidance documents and related compliance requirements. Although such documents and requirements are numerous of increasing complexities, their content remains subjective and arbitrary in nature, and in many cases scientifically invalid and contradictory. This has given birth to fantastic illusionary practices of quality assessment named as pharmaceutical science, regulatory science, risk management (science), quality management (science), compliance, inspections, etc. However, the fact remains that no one is monitoring the quality of products nor are they able to monitor it, as the “quality” is an unknown or undefined entity. This is a fanciful example of an “illusionary science” promoted and practiced by most “experts” in the area causing delays and hindrances in obtaining and manufacturing of products in a cost effective and timely manner.
Such an impeding, confusing and illusionary situation can be eliminated, if one would work with defining the quality of a pharmaceutical product which by itself is relatively easy to define as suggested here (link). It is therefore humbly requested that regulatory authorities take note of this request and consider including a definition of a quality product so that needed quality products could be manufactured in a timely and efficient manner.