Monthly Archives: June 2016
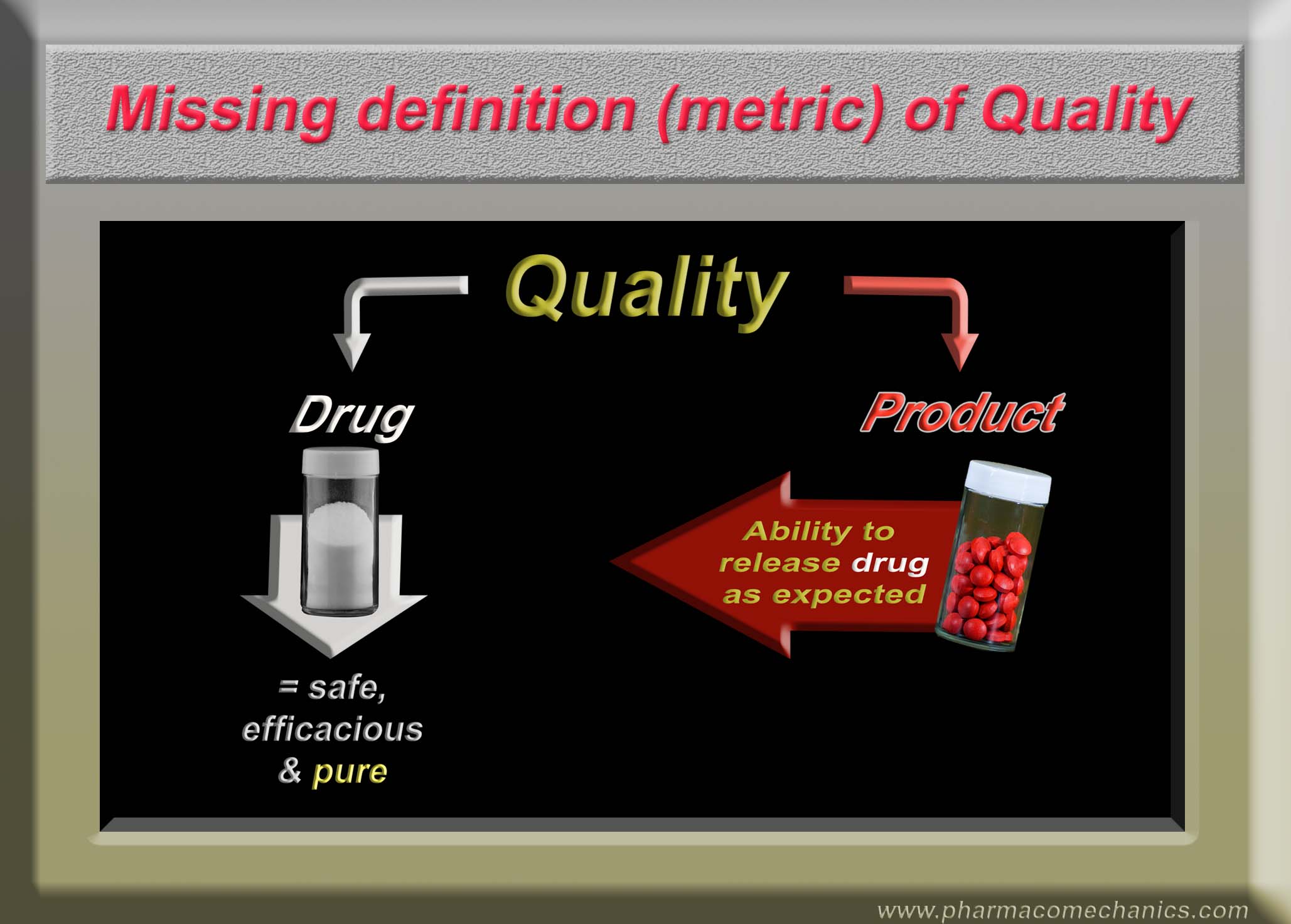
Quality of Pharmaceutical Products
link (1)
Let me be bold and direct!
Dear regulatory and pharmaceutical scientists, please note that all dissolution tests as described in the USP and/or FDA database lack scientific basis and are invalid for assessing any of the drug product characteristics including quality. The recommended testers and associated methods have never been validated and/or qualified for the intended purpose. These tests are conducted as a regulatory requirement, which forces people/industry to use the testers and the methods resulting in false claims regarding quality of products.
To be scientifically valid, the tests must be product independent and testers must be validated and qualified for dissolution testing of products for human use. Please pay attention! (links for further details 1, 2)