Monthly Archives: October 2011
Assay and Content Uniformity (CU) based on dissolution testing (Poster Presentation)
This week I presented a poster at the AAPS 2011 (Washington, DC) meeting describing a study to assess the Assay and CU from a dissolution test. A copy of the poster is attached (link). I hope that you will find it useful and helpful for your own work.
Simplifying Pharmacopeial Tolerances – An Example Based on USP Tolerances for Diltiazem ER Capsules
USP diltiazem (ER) monograph describes 15 sets of tolerances or by definition “the permitted variation in the results (link)”; four sets for 12-hour and eleven sets for 24-hours products. Figures 1 and 2 present these tolerances in a profile format by using the average values of the ranges described. The first and last tolerances where ranges are often described as NMT (not more than) or NLT (not less than) respectively, averages are calculated using zero and NMT values and NLT values and 110 (highest expected percent dissolved from the CU requirement).
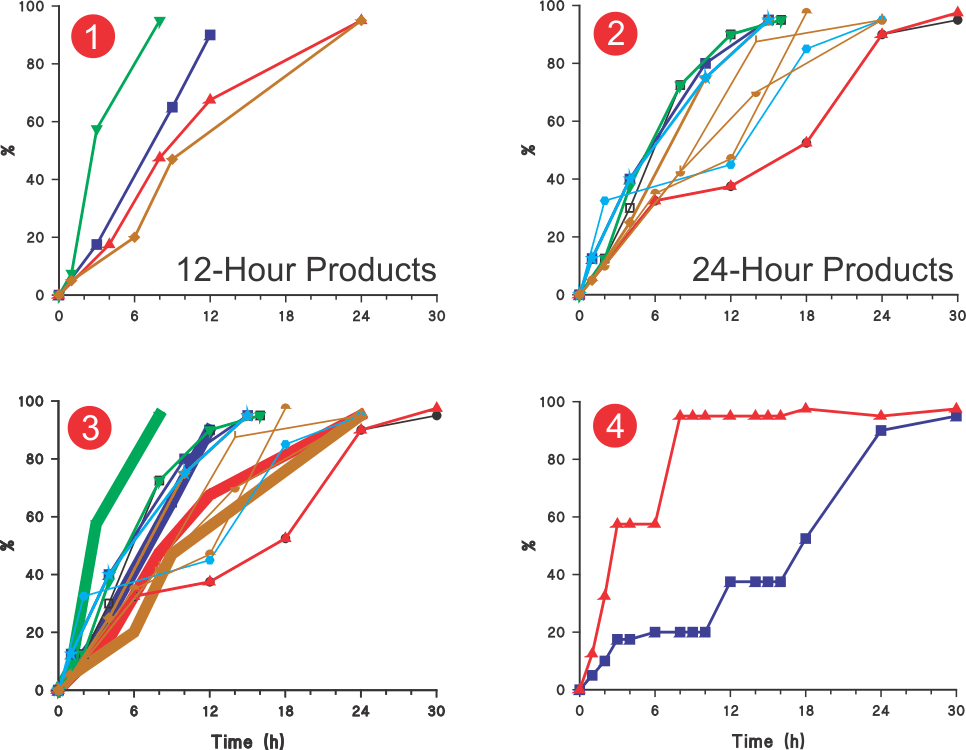
In short, these profiles represent acceptable variation in dissolution characteristics for presumably good quality (without a clinical concern) diltiazem products which are permissible for sale. Although, the monograph describes the tolerances for 12- and 24-h products separately, in most cases these tolerances appear to overlap, as shown in Figure 3. Therefore, it would be safe to assume that, from the pharmacopeial perspective (in vitro testing), both of these types of products will show similar dissolution characteristics!
In most cases, tolerances are based on separate dissolution test conditions. Therefore, it is not possible to ascertain whether the dissolution characteristics are reflective of a product characteristic or because of the experimental conditions used. Therefore, it may not be possible to establish the quality of the product.
On the other hand, if such variations in drug dissolution (release) results are acceptable then the tolerances can be simplified by representing these only with one set of tolerances (upper and lower limit profiles) as shown in Figure 4. This would avoid the current complex and resource intensive practices of setting individual tolerances with no apparent advantage. In addition, it will also help in establishing underlying expected variability in dissolution results.
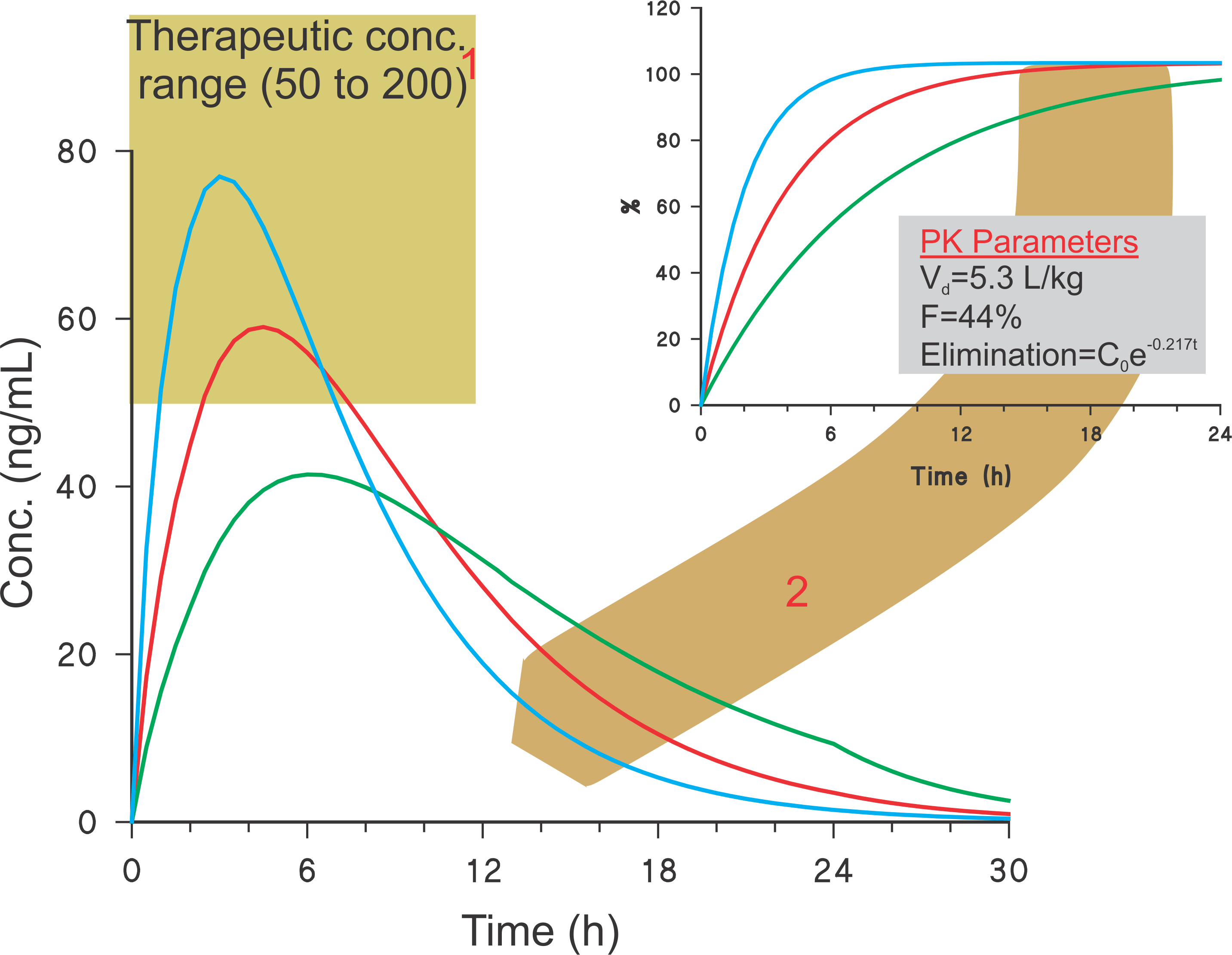
Estimation of blood levels – Example: 120 mg diltiazem ER capsules
Defining a dissolution apparatus
In general an apparatus means a machine having a specific function. In our case, a dissolution apparatus means “a machine which may be used to determine dissolution characteristics (function) of a drug product such as a tablet or capsule”. This function (dissolution) can only be achieved if the machine is able to provide thorough but gentle (low rpm) stirring and mixing within a vessel. Another way of saying the same thing is that the machine should provide thorough but gentle product/solvent interaction at low rpms, ie, less than 100.
 The reason the machines shown above may not be considered as dissolution apparatuses is that they do not provide an appropriate and required product/solvent interactions, thus drug dissolution. All these machines required very high speed (rpms) for mixing. At lower rpms the test products often lie around stagnant, thus incorrectly indicating limited or no dissolution.
The reason the machines shown above may not be considered as dissolution apparatuses is that they do not provide an appropriate and required product/solvent interactions, thus drug dissolution. All these machines required very high speed (rpms) for mixing. At lower rpms the test products often lie around stagnant, thus incorrectly indicating limited or no dissolution.
A critical requirement for a dissolution apparatus is that it should be capable of providing stirring and mixing at low rpms and it must also avoid stagnation of the test product.
Developing and validating a dissolution method – suggestions for simplification
Method development and validation is a critical and important part of drug dissolution testing and is required for an appropriate product evaluation. It is a common practice and often time confusing and frustrating exercise. In addition, these exercises are often described by different names such as developing QC/QA, bio-(relevant) and/or discriminatory methods. In additions, these method development exercises are further complicated by requiring different types for each drug and product. In fact, a single type of product of the same drug, in particular ER, can have multiple methods.
On the other hand, however, if the methods described in literature are critically evaluated, it would be quite obvious that there are not significant differences in these methods. The majority of the methods described in literature are based on USP apparatus 1 (Basket) or 2 (Paddle) set mostly at 100/75/50 rpm with water or some form of buffer, having a pH in the range of 5-7 as a medium. This experimental part is followed by usual method validation steps. Confusion and frustration arise from the question of how to select a particular set of experimental conditions (apparatus, rpm and medium). Unfortunately, there is no rationale or scientifically valid approach available for selecting the required set of experimental conditions. Formulators or analysts are often expected to make their own judgment calls, which causes the confusion and frustration.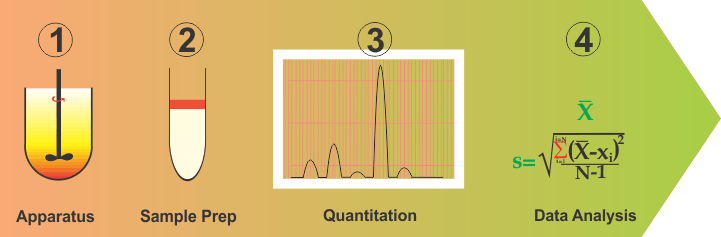
Although, complex and difficult as this situation may be, one should be able to address this problem by simplifying and breaking down into parts the method development and validation exercises. In this regard, one can break this down into four distinct parts: (1) Apparatus containing medium; (2) Sampling or cleanup step; (3) Quantitation and; (4) Data analysis (or validation). Continue reading