Using Crescent-shape spindle for different dosage forms and suggestion of a “standard” tablet product
The following is a response to a question on LinkedIn Forum, which, I thought, would be valuable to the visitors of this blog as well. I hope you will find it useful. Saeed
Question:
Saeed: Aside from the fact that cGMP is commonly understood as the current norms of industry, would you advise on how your approach would apply to other dosage forms? The maceration/muddling/contact mixing apparatus you’ve designed might be able to replace paddles and baskets for tablets. What would be the solution for testing of sublingual and transdermal films using paddle over disk or cylinders? If you feel that performance verification is necessary (running a “standard” product), wouldn’t you provide a better service to industry by developing a better “standard” product than the Prednisone PVT tablets?
My response:
Louis: Excellent questions. I personally believe that dissolution testing is, and should be, for oral products only. The dissolution tests mimic volume/space, liquid, stirring/mixing, and temperature of the GI tract, in particular intestinal. Other than stirring/mixing environment these apparatuses (paddle/basket) are fine. As the paddle/basket do not provide appropriate stirring/mixing but stagnation, and also highly variable results because of variable settling positions of tablets/capsules at the bottom vessel, they do not mimic GI environment appropriately, thus are not suitable for dissolution testing. Crescent shape spindle addresses these flaws therefore offer better mimicking of GI tract and thus dissolution testing. The reason I explained this is that there is science and logical thinking behind the development and suggestion of the crescent shape spindle. It is not like that OK if paddle/basket does not provide expected results try something else without looking and addressing the cause of the problem.
With this background, my view about sublingual products is that you may try and it might work. As I do not have experience working with such products I can only envision that the crescent shape spindle might provide faster dissolution results than what one would observe in vivo as in in vivo tablets move slower than in vitro with the crescent shape spindle. But then one should be able to conduct dissolution at a lower rpm say 5 for such products. Certainly, you should try.
Concerning patches or other dermal/topical products, I do not think that dissolution testers (paddle/basket or crescent shape spindle) should be used at all. There is no link or simulation of in vivo environment here for such products i.e. science does not support such testing. However, if one insists on using it for some reason, then one may use the same setup for patches with disk and hang it in the vessel from the lid of the vessel or tie it to the rod of the spindle. Most likely results will be better than using paddle/basket as crescent shape spindle provides much better mixing environment.
Concerning developing “standard” tablets, I would not develop standard tablets, like PVT which in my view was a wrong concept. Tablets do not, and cannot, reflect deficiencies of the individual physical parameters of testers and/or tests. The “standard” tablets are only be used to show that, AFTER meeting all the physical specifications collectvely, the tester is “performing” well, i.e. capable of providing expected dissolution results of a PRODUCT for human use (not just any tablets or product). Therefore, I have some ideas of picking up a product, as a “standard” in this regard. I am looking for a product manufacturer who would be interested in working with me for the selection of such a product. So, anyone interested in such (lucrative) work or market, please contact me. I believe it would be a small and short project with big financial reward and service to pharmaceutical industry.
I hope I have answered your questions adequately. Let me know if you require further details.
Non-GMP compliant dissolution testers but no warning letters!
See here for details (1)
Please Pay Attention!
See here for details (1)
Product Quality Metric (do not confuse it with drug/medicine quality)
See here for details (1)
ICH – Quality Guidelines
link (1)
A Quality Product – Please Define!
link (1)
Quality of Pharmaceutical Products
link (1)
Let me be bold and direct!
Dear regulatory and pharmaceutical scientists, please note that all dissolution tests as described in the USP and/or FDA database lack scientific basis and are invalid for assessing any of the drug product characteristics including quality. The recommended testers and associated methods have never been validated and/or qualified for the intended purpose. These tests are conducted as a regulatory requirement, which forces people/industry to use the testers and the methods resulting in false claims regarding quality of products.
To be scientifically valid, the tests must be product independent and testers must be validated and qualified for dissolution testing of products for human use. Please pay attention! (links for further details 1, 2)
Essential requirements for a bio- or clinically relevant dissolution tester/method
The tester must be shown first that it is capable of providing relevant dissolution results of a product with low agitation (stirring) environment i.e. it is capable of providing expected and consistent dissolution results with acceptable variability e.g. 10 % CV or RSD. (Physical or mechanical requirement)
The dissolution results obtained must relate to the physiological release characteristics of the product using a physiologically simulated medium (solvent) i.e. a fast drug-release product to provide faster dissolution results while slow drug-release to provide slower drug dissolution results without changing the experimental/testing conditions i.e. test/tester must be product independent. (Physiological requirement)
Since, none of the currently recommended testers and/or methods, including pharmacopeial and/or regulatory, meets the above mentioned requirements; they cannot be considered as bio- or clinically relevant testers or methods. In addition, since none of the currently recommended testers have been shown to meet condition #1 (above), these cannot be considered capable of providing bio- or clinically relevant tests/methods. Suggesting otherwise would be a scientifically invalid practice or claim.
For further discussion on the topic please follow the link.
Please do yourselves a favor:
Quality vs efficacy and safety of pharmaceutical products
link (1)
Assessing quality of pharmaceutical products!
link (1)
Dissolution Testers – A Mystery
link (1)
Drug Dissolution Testing – Illusion!
link (1)
Scientific and GMP violation!
link (1)
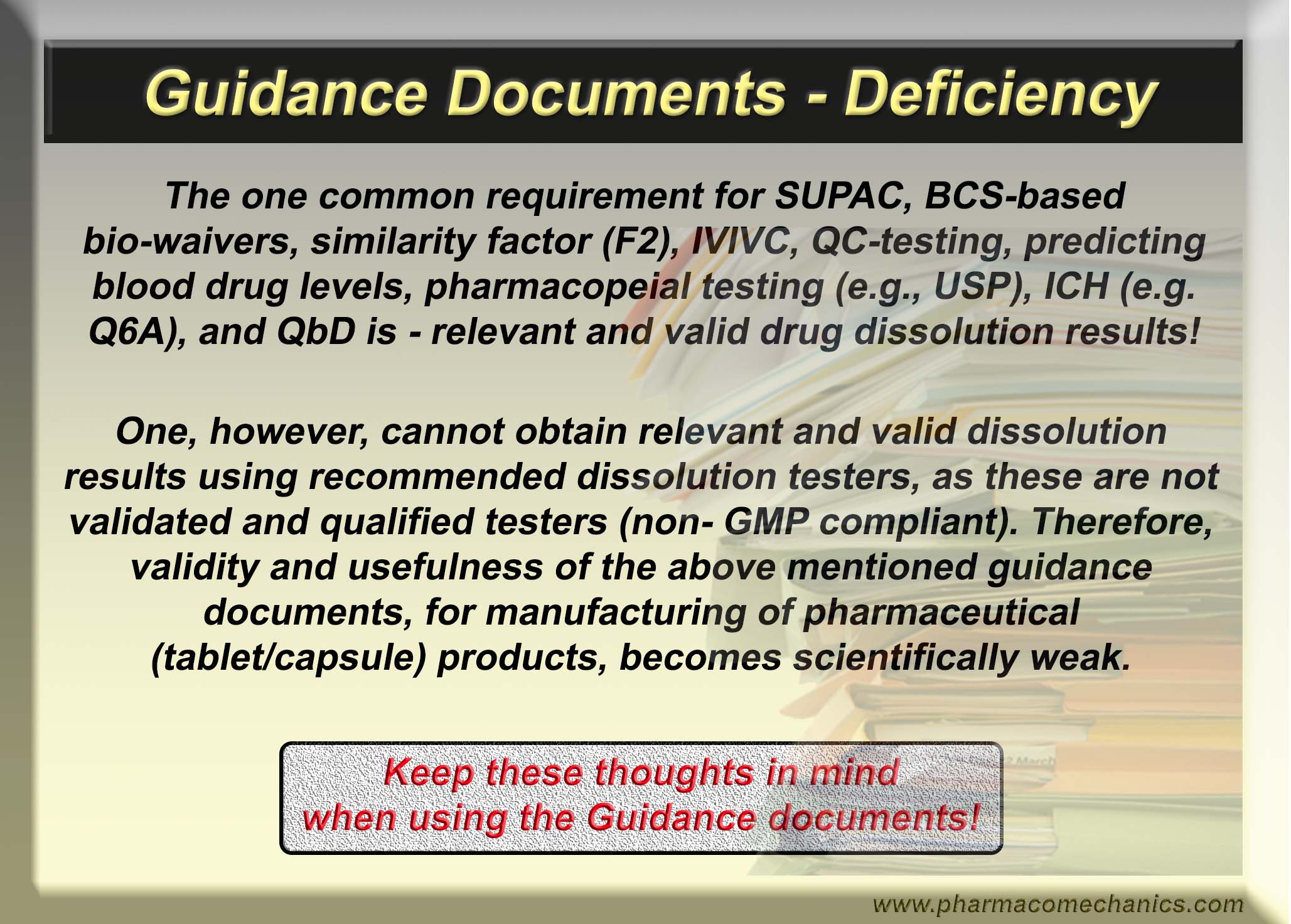
Guidance Documents – Deficiency
f2 – Similarity Factor
For details, please see here
Sink Condition
For details, please see here
“Improving solubility and absorption characteristics of drugs”, is such a practice scientifically valid or accurate? Maybe not!
It is quite often described in literature (e.g. see here) that drugs may cause problems due to their low solubility and/or low absorption characteristics through the GI tract. To address such issues, often it is suggested that solubility of drugs may be improved which would increase the absorption, thus would provide better drug and/or product safety/efficacy profiles.
In this regard, it should first be noted that the low solubility of a drug by itself should not be the cause of a concern for efficient absorption, as the body, in particular the GI tract, does not require high solubility of a drug, or complete dissolution of drug at any given time. As explained previously (link) continuous/repetitious process of absorption in the GI tract take cares of the low solubility and/or dissolution aspects of the drugs.
Furthermore, it should be noted that both solubility and absorption are inherent or intrinsic properties of a drug and cannot be changed. It is just like molecular weight, melting/boiling-point, density/specific gravity and spectrum (e.g. UV) of drugs, which are intrinsic properties, and identify a compound or molecule. If any of such characteristics differ or change then that means that compound or molecule is not original and morphed into something different. Therefore, if solubility and/or absorption of a drug are changed, intentionally or unintentionally, then the drug is not the same molecule which body would see. Its absorption and/or pharmacokinetic (including metabolism/elimination) profile may be different as well. In reality, it would be a different or a new drug itself with its own efficacy/safety profile. Therefore, one should be careful in seeking improvements in solubility/absorption profile of a drug which may result in a potential new compound/drug, and may require separate safety/efficacy profile assessment.
On the other hand, if a physical mixture is made with other components, by simple mixing or complexation, which would dissociate within the GI tract, then the drug will behave as the original. Thus, such “new” formulations would not, at least should not, be of any help or use in changing solubility and/or absorption characteristics of the drugs.
Compliant/approved vs quality drug products
A suggestion for the New Year
Use care in using dissolution testers, in particular the paddle and basket. These testers have never been validated and qualified for their intended use (link), therefore, interpretation of the results obtained would most likely be misleading. This would be particularly true for the assessment of quality of the products, which, at present, by itself is an undefined parameter and objective (link).
Often people promote the use of current practices of dissolution testing as a regulatory compliant requirement, which to some extent is correct but unfortunate practice. However, my view is that such regulatory requirements will also be discontinued soon. Scientifically speaking, it is highly unlikely, if not impossible, that such practices can continue knowing that these testers are non-qualified and/or non-validated (a violation of GMP requirements).
Wish you all, the best of luck and success in your professional and personal lives in the coming years.
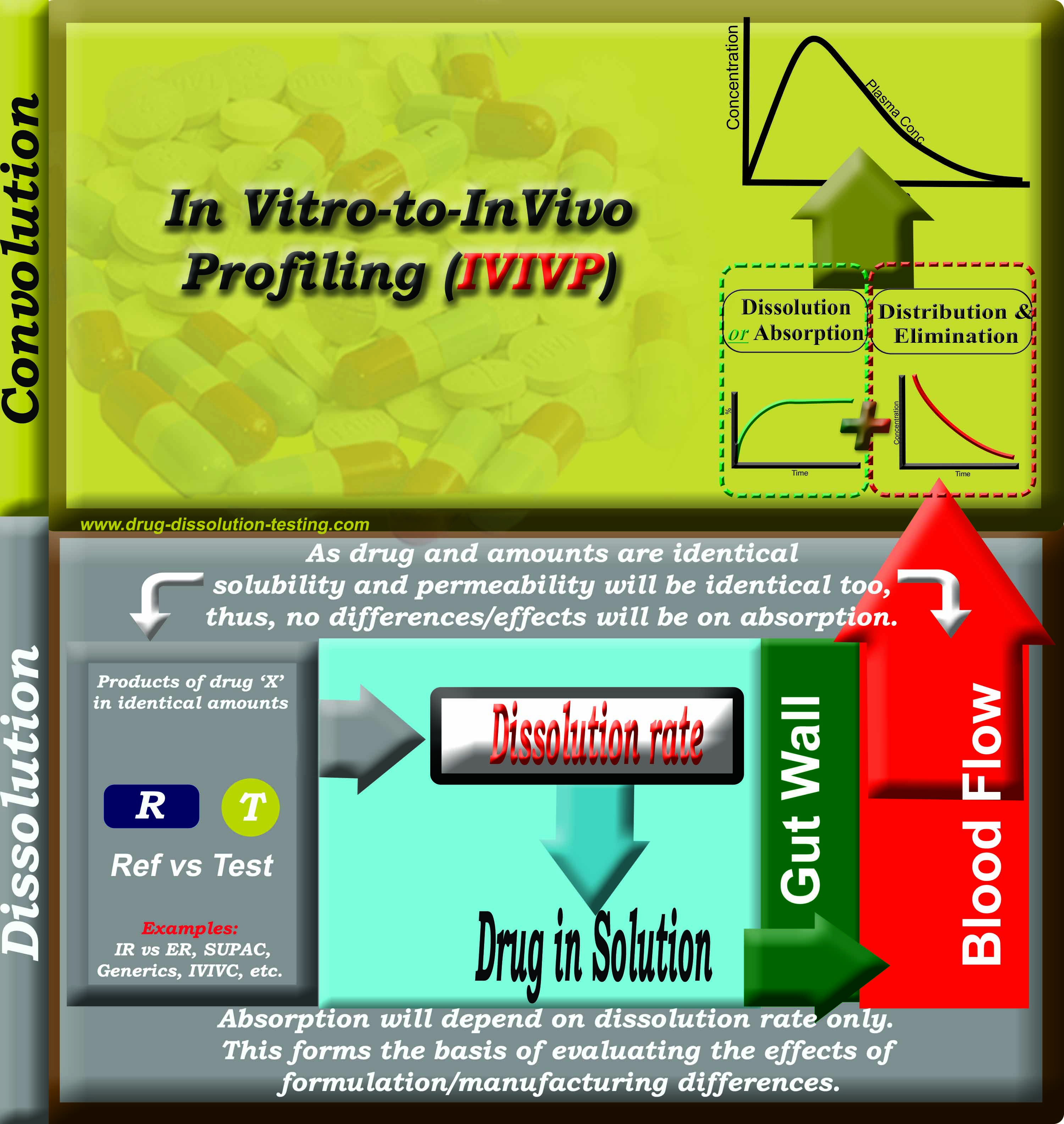
Schematic representation of the principle of formulation/product assessments using IVIVP (in vitro-to-in vivo profiling) approach
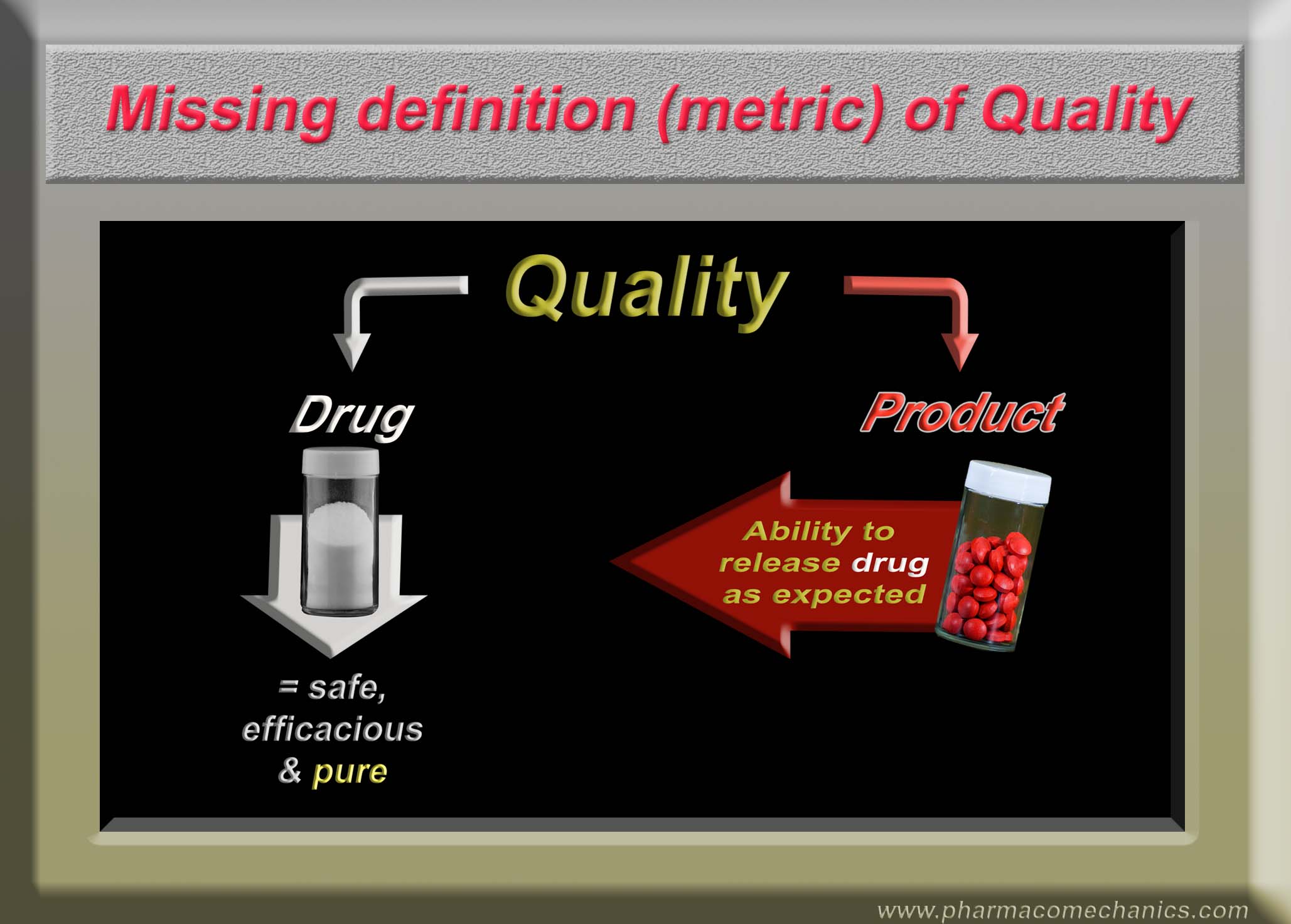
The missing quality definition or metric
This definition/metric needs to be included, as an assessment of practically all other factors such as safety, efficacy, manufacturing and its design and processes etc., are secondary to it. In addition, such a metric can easily be explained to the consumer/patient. For further discussions on the topic, please see the link.