John OSullivan
Saeed Qureshi
Author Archive
Does anyone have the following virus/COVID-related information? Please share.
A reference or link:
- of having seen or possessing a pure virus’s physical specimen, not an isolate or a picture of a mixture/gunk (commonly referred to as cell culture).
- To an experimental/scientific study showing that the suggested vaccines kill the virus in humans. A physical sample of the dead-virus in the presence of a vaccine or its derivative.
- Showing that face-masks (any type) stop or significantly reduce the virus’s passage (in or out). The study must include a virus sample (not droplets or aerosols only as a virus substitute).
Dear scientists, experts, and authorities, please help. These should be easy questions to answer with all the fundings you have obtained and claims you made. Otherwise, what is the point?
.
.
PS: FYI, the answer is, such links or studies do not exist.
Zoom Meeting with a UK Panel
A couple of days ago, I participated in a Zoom meeting with a UK panel (physicians and others) on the situation (link). The meeting was very long; however, if interested, consider watching at 12:47 and 58:15 (in the latter part, I did not participate as I was not aware that the meeting continued after the disconnect).
It indeed appears that confusion is there, or created, to gain some advantages. Also, it remains questionable practice, at least to me, to treat people, the healthy ones in particular, with a new treatment, which is still in a developmental phase.
As noted here (link), I believe that we may be seriously in a misdiagnosis and mistreatment situation. This situation should not continue.
(Link to the first part of meeting/discussion)
More bad news concerning virus isolation and PCR test
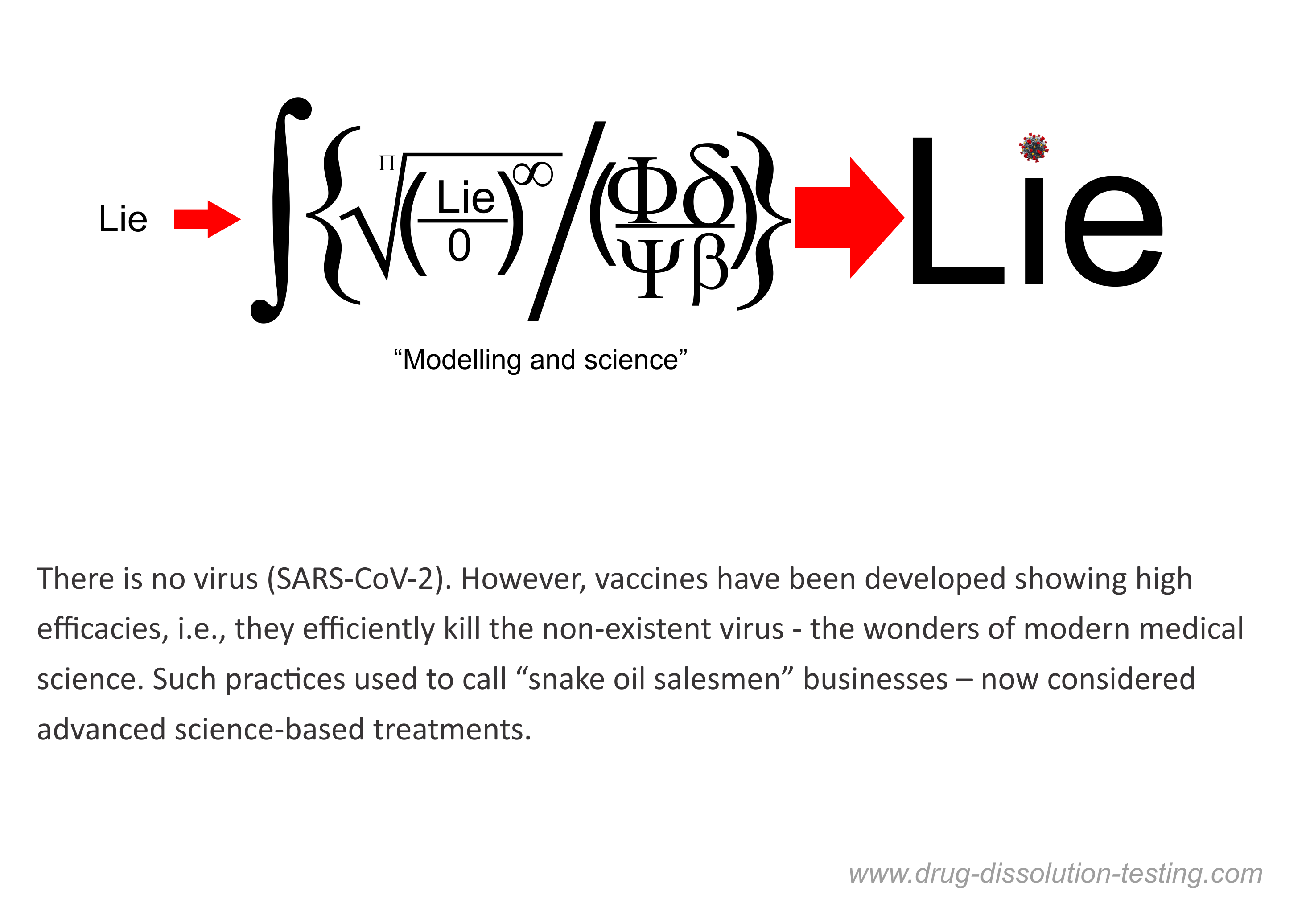
I have been highlighting for some time that the virus (SARS-CoV-2) has never been isolated or positively identified [1,2,3,4]. Therefore, it cannot be claimed that the virus exists, and by extension, the story of the COVID pandemic cannot be considered science-based or factual.
This idea of non-isolation of the virus has been gaining traction. A recent report further emphasized that the SAR-CoV-2 has not been isolated, along with any other viruses in the coronavirus family [5].
The use of the word “isolate,” with the implied meaning or representation of the term isolation of the virus, misled everyone, including physicians, scientists, experts. They assumed that the virus or viruses are real and have been physically isolated.
The article mentioned [5] above also clearly discredit the PCR test’s relevance and usefulness, as I have been saying for quite some time [6,7,8]. A prestigious international expert on the subject, Dr. Stephen Bustin, is quoted, describing both the arbitrariness of criteria for RNA results and choosing the number of cycles leading to anyone testing positive for COVID. The mentioned article [5] discusses the flaws of PCR tests and methodology for its use as a diagnostic tool.
On the other hand, as I have repeatedly described, the PCR test is a chemical test that has never been validated for its intended use. It is a blatant violation of the fundamental principle of science-based chemical/clinical testing. Such a test can never provide relevant and valid results. Surprisingly, such testing is accepted by the regulatory authorities, including the FDA. The test and its associated results should be withdrawn immediately.
In short, claims of isolation of the virus (SARS-CoV-2) and the PCR test are shown to be scientifically invalid and irrelevant.
Saeed Qureshi
Author Archive
Bye Bye – Bioequivalence testing? Long live drug dissolution testing! —– #2 (gift from heaven)
Yesterday, I posted my view on the recent FDA guidance documents for chloroquine and hydroxychloroquine (link). I do not think people realize the long term impact of this development where BE studies have been replaced/substituted with drug dissolution testing. Let me explain:
- Saying that guidances are product specific is not correct, because chloroquine and hydroxychloroquine are drugs not products. Products are tablets, or capsules, with often unknown and proprietary composition of a drug, excipients and manufacturing attributes (i.e. formulation and manufacturing attributes). Hence, guidances cannot be product specific as assumed or suggested.
- A drug dissolution test is conducted for products not drugs. As product attributes are mostly unknown and propriety, as noted above, hence a dissolution test (or guidance) cannot be product specific but has to be independent “standard or universal”.
- Furthermore, it is to be noted that in principle product specific guidance concept is an invalid concept. Drug dissolution testing is a scale used to measure dissolution characteristics of a product. By definition it (scale) has to be independent to the tested items. Point being that the guidance documents cannot be restricted one or two drug products. These have to be applicable to ALL highly soluble drug products. It would not be possible for authorities, at least scientifically, to defend restricting to only one or two products. This decision could easily be challenged and won.
- In addition, such a decision cannot be one time decision, as many believe, may it be taken under an emergency situation. It would not be possible to withdraw such a decision once taken i.e. if dissolution test alone can provide quality assessment of the products, then why would BE studies be needed and required on what basis especially when BE studies are known to be irrelevant (link).
- This new development is a gift from heaven for the underdeveloped countries where because of lack of BE studies, products and their manufacturing have always been labelled inferior. However, with the requirement of dissolution testing only, everyone can manufacture and promote (for local and/or international markets) their quality products with confidence.
Keep these thoughts in mind and proceed accordingly.
Bye Bye – Bioequivalence testing? Long live drug dissolution testing!
Recently FDA provided 1- and 2-pager guidance documents for chloroquine and hydroxychloroquine, respectively (link).
The most interesting part is that one can get product approval based on dissolution testing alone. This is what has recently been suggested in one of my recent published articles i.e. products (“quality”) assessment can easily and accurately be established with drug dissolution testing alone (link). There is really no need of conducting bioequivalence (BE) assessments. These (BE) assessments procedures have never been validated of the intended purpose. In fact BE are scientifically invalid and can provide false conclusions and assurance about product quality. In addition, such testing exposes healthy human volunteers to highly potent chemicals under the disguise of medicine development.
On the other hand, switching to dissolution testing alone using currently recommended USP apparatuses is not valid either, at least scientifically. The recommended apparatuses are non-GMP compliant and can provide false and irrelevant results because of their intrinsic design and operation problems. Simpler and scientifically valid options are available and could be used (link).
Is Coronavirus really causing abnormally higher number of deaths?
Mortality in the United States, 2018 (as of January 2020, link).
“The age-adjusted death rate decreased by 1.1% from 731.9 deaths per 100,000 standard population in 2017 to 723.6 in 2018.” i.e. death rate is about 0.7236%
For the USA, having population of 331 million (link), normal/standard death (attrition) rate should be 199,593 deaths/month. Now compare this number with the reported number of deaths caused by Coronavirus pandemic, which are 21,435 in about a month’s time as of April 12, 2020 (link) which is far less than normal/standard death (attrition) rate.
The death rate, therefore, does not appear to support the thesis that the pandemic is killing people with abnormally high numbers.
Response to a recent query: Manufacturing of hydroxychloroquine and seeking help from the authorities
Just this morning I received the following query (my response is included as well) about the manufacturing aspect of the above mentioned drug product. It is hoped that authorities will take a note and address the issue faced by the industry to manufacture this important pharmaceutical product as per my year and half old Citizen Petition (link).
Query:
“Regarding dissolution of HYDROXYCHLOROQUINE SULPHATE TABLETS, the disso medium specified in IP & USP is water. The formulation sometimes fail to conform to IP and even USP parameters
I have tried replacing water with 0.1 M hydrochloric acid as dissolution medium and achieved a disso of above 90 %. I know the api is water soluble, but since this is an instant release formulation and the approximate pH of stomach is being maintained in disso medium, can we recommend change of disso medium from water to 0.1 M H Cl to IP & USP.
Response:
Scientifically speaking water is an appropriate dissolution medium not the HCl (link).
In reality, your suggestion of changing the medium from water to HCl is for obtaining desired dissolution results, which is neither scientific nor logical.
In general, the issue you are describing is not of medium choice but choice of the dissolution apparatus. USP apparatuses are known to provide slower and irrelevant results; most likely you are observing this flaw. Such an issue can only be addressed by changing the tester not the medium, rpm etc. Considering USP apparatuses flaws and limitations, I have proposed a new stirrer, precisely to address this issue. Perhaps consider using this suggested spindle and simpler dissolution method not only for this product but also avoiding future issues with the use of USP apparatuses. There is a stronger argument available in using an alternate dissolution tester than changing dissolution medium i.e. the USP apparatuses are non-GMP compliant (link, link).
I hope you will find suggestion useful and best of luck.
PS: Please request the authorities, in particular FDA and USP, to withdraw the requirement of using non-GMP (i.e. non-validated/non-qualified testers/methods) and allow the use of scientifically valid testers and methods to develop and manufacture urgently needed pharmaceutical products.
My recently published book chapter (details below) …
… provides in depth discussion on establishing and monitoring quality of pharmaceutical products in particular tablet and capsule. I hope you will find the chapter useful.
Chapter title: “Quality” of pharmaceutical products for human use – underlying concepts and required practices, published in
Drug Delivery Trends: Expectations And Realities Of Multifunctional Drug Delivery Systems Volume 3 Edited by Ranjita Shegokar, PhD., Available from Amazon (March 18, 2020), link.
Saeed Qureshi
Author Archive
(Video) Virus, COVID, pandemic, vaccine, and testing: fiction, not reality or science!
Click on the picture to play the video
If you prefer to read from the text, it is available here (link)
Please donate (by clicking the button below). Thanks.
Did COVID cause “excess deaths?”
COVID-19 is a recently-labeled illness presumably caused by a virus named SARS-CoV-2. The illness is considered contagious, i.e., assuming that the virus spreads from person to person directly or indirectly. It is believed that COVID-19 caused the pandemic resulting in a large number of deaths.
This article reflects an exercise in summarizing the data in seeking a potential trend from COVID-19 deaths to guide addressing the pandemic issue. (Continue here)
Getting out of the coronavirus pandemic – legitimate and scientifically valid approach
People should realize that reducing the Ct (cycle threshold) of the PCR test, as some suggest, may reduce the number of test-positive results. This will certainly help reduce the so-called pandemic—a trickery approach to bring the pandemic under control that never existed in the first place.
However, the fact remains that the PCR test is scientifically invalid, no matter how low Ct value one would set. To have a valid test, it requires to meet four well establish validation criteria: (i) specificity; (2) selectivity; (3) reproducibility; and (4) use of independently characterized reference standard. The first three criteria cannot be met if the reference standard (the #4) is not available. If the claim is that the PCR test monitors the presence or absence of the virus. Then reference virus must be available in its pure form. On the other hand, if virus RNA or its fragment is to be monitored, RNA or its fragment must be available as the reference standard and positively shown to be extracted from the virus (further details here).
Considering that the tests or testing are based on confirming the RNA sequencing only from an aliquot of media/culture/isolate, hence is a valid test, is pure nonsense. Such tests have no relevance to the virus, illness, and pandemic monitoring, and these PCR tests must be stopped immediately and preferably withdrawing all related results/data as false. This will be the legitimate and scientifically valid approach for getting out of the pandemic state.
It is hoped that science will prevail.
COVID cannot spread – with or without a mask or social distancing
COVID-19 is an illness presumably caused by a virus named SARS-CoV-2. The illness is considered contagious, i.e., the virus spreads from person to person directly or indirectly. The virus is commonly viewed as a particle. For it to spread, the particle has to exist.
The problem is that such virus particles do not exist because no one has isolated them or positively identified them (details here).
If something does not exist, how it will transfer from one person to another. It can’t.
Therefore, wearing masks or keeping a distance becomes irrelevant. It is that simple!
When “isolation of a virus” is not the isolation
As a part of a LinkedIn discussion (link), I provided the following (below) response to a query. The visitors of this blog may find my response informative and useful as well.
_________________________________________________________________________________________
Steve:
Thanks for your kind words about my article and also for asking the questions.
First, to be clear that I am not a microbiologist or virologist. I am a chemist and have worked in the pharmaceuticals area for 30+ years (as a scientist with Health Canada). I gained significant experience and expertise in critically evaluating pharmaceutical products.
Regarding the claim of virus isolation, I am saying that the experiments microbiologists/virologists perform and describe, such as virus characterization, identification, belongs to the chemistry discipline. However, the chemistry work has not been conducted accurately; hence claims made are incorrect.
There are many ways to describe and explain the inaccuracies. One of which is that of isolation of a substance, in this case, a virus. If one needs to isolate a virus, one must go through multiple steps, such as extraction, purification, identification, and structure determination, resulting in a pure sample of the virus. Nothing of this sort has been done for the virus isolation, in particular, SARS-CoV-2. Therefore, it cannot be said that the virus has been isolated and identified, or even it exists.
On the other hand, microbiologists and virologists (among others) work with a modified definition and description of the term isolation, for their purpose, as taking a swab sample (i.e., separating virus from the host). That is not a good scientific practice. Therefore, in reality, a virus never gets isolated in its true or pure form.
On the other hand, to appear scientific, the DNA/RNA sequencing is considered as a claim of “identifying” the virus in a swab sample without truly “isolating” it (we chemists call it a clean-up step). Some chemical steps using enzymes (commonly known as polymerization, again a chemistry step) are conducted, followed by taking some pictures of the soup with an electron microscope. Observing spherical bodies with spikes are considered to reflect the existence of coronavirus.
From this soup (note everything is from the soup, nothing from pure virus), DNA or RNA or its fragments are extracted to establish their sequences. There is no evidence that this DNA or RNA is from anything specific, including the virus – it is an assumption. Based on computer analysis and comparison with previously obtained “reference” sequence (usually obtained from WHO depository), which is also “isolated” in a similar manner (without isolating the virus), virus existence is established. If the sequence did not match the “reference” sequence (not a virus), it would become a new virus or new strain.
The publications, links you provided all follow the same or similar protocol as I summarized above. I have critically reviewed two such publications, one from Australia (the one you noted in your post as well), the other from the USA (CDC), where I explained: “science” behind “isolation” of the virus. Links are provided (link1, link2) please have a look.
I have been arguing for some time that nowhere I can find an isolated virus, so why people keep claiming isolated virus. My recent discussion with a microbiologist made it clear that the virus has never been isolated but misrepresented by incorrect definition of the word “isolation.” That makes it clear why I could never find the sample and specimen of the pure virus because it does not exist. One may imagine my shock after hearing this – so I wrote the article.
I hope I answered your query adequately. Otherwise, let me know. I will explain it further. I like to make another point, without going into technical details, the sequencing (chemistry) part is pretty iffy. It is well known to the people in the area that sequencing steps can produce highly unpredictable results. The PCR test, which in reality is based on sequencing, suffers this weakness. Hence one sees so many false positive or negative outcomes that make the lack of “virus existence” claim even stronger. (edited)
Dear regulatory authorities:
“Regulatory (pharmaceutical) science” – lacks logic as well as science!
One cannot establish quality of anything without knowing or defining it first. This is simple logic!
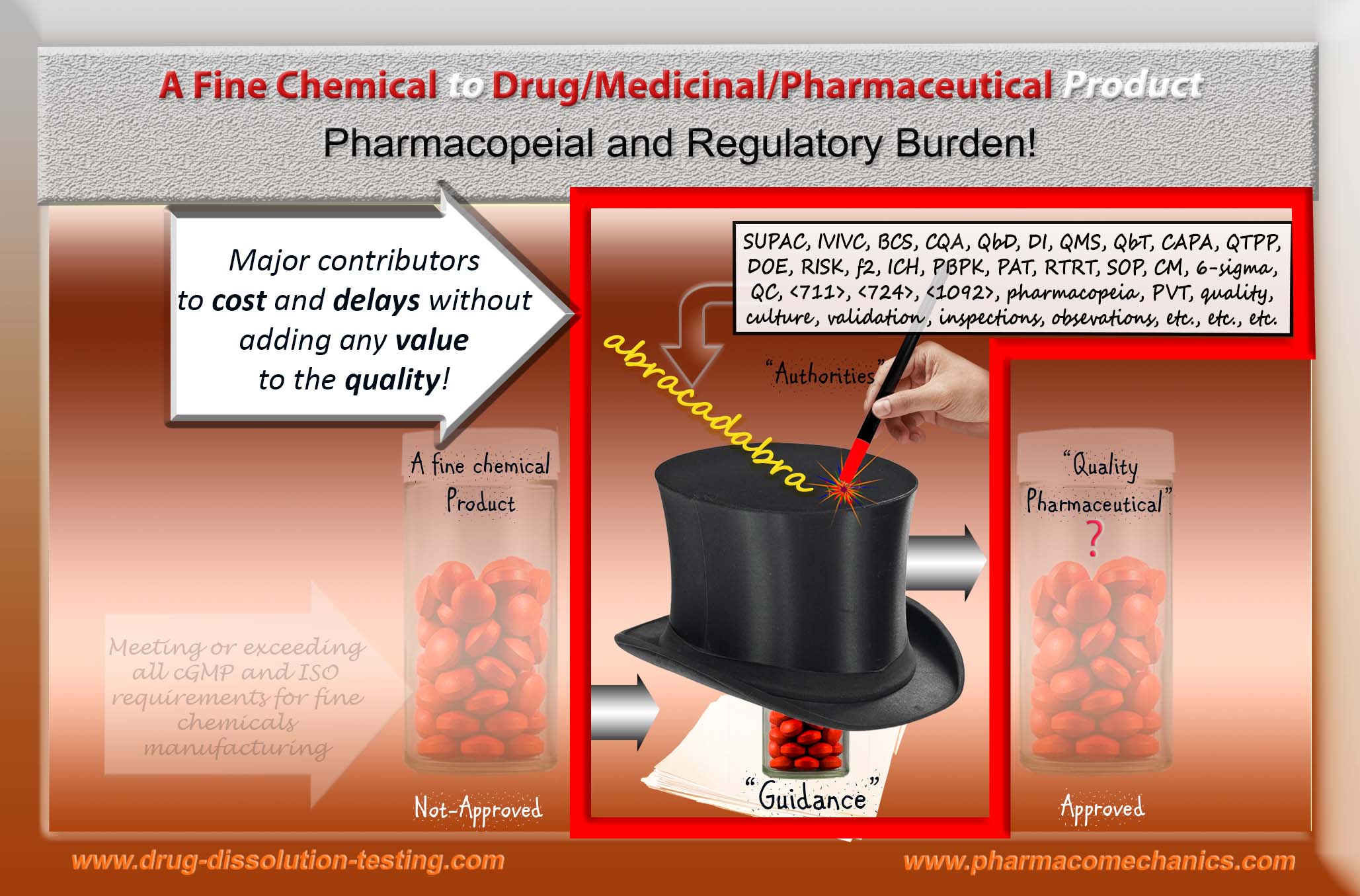
Regulatory authorities including pharmacopeias, however, have been trying to prove this logic wrong! That is, they have been making claims of establishing and monitoring quality of pharmaceutical products such as tablet and capsule – without knowing or defining it. Obviously, they will fail and have been failed!
Logically and/or scientifically, none of the products (approved or otherwise) available on the market can be considered of quality. Guidance and compliance-based system, along with the plant inspections, is a thick smoke screen hiding the reality and hindering the progress.
Please, define a quality product and set the standards and specifications accordingly so that appropriate quality products could be manufacture and monitored. For further detail, please see here.
Selecting medium for drug dissolution testing: Please pay attention to the principles of science and the laws of nature
Considering solubility characteristics of a drug in the stomach (i.e., pH range of 1 to 3) are pretty much useless from the perspective of absorption of a drug. Even if a drug gets dissolved in the stomach, it will be precipitated out in the intestine if it has lower solubility at a higher pH.
For absorption purposes a drug must dissolve, not necessarily completely but in some quantity, in the intestine where pH ranges from 4.5 to 7. Drugs get absorbed in steps in the intestine by continuous extraction process thus complete dissolution of drugs, at any given time (so called “sink condition”) is not necessary.
Moreover, dissolution characteristics are not usually determined for a drug – dissolution tests are conducted to evaluate products. The choice of medium is linked to the physiological environment of the GI tract not to the drugs or products. Please pay attention to the principles of science and the laws of nature. For further detail please follow the link (http://www.drug-dissolution-testing.com/?p=1749).
Simulation and modelling practices for establishing quality of pharmaceutical products – valid intentions but invalid outcomes
Dear experts:
In the area of simulation and modelling, including developers of commercial software for such, note that I may not be able to argue with you regarding your methodologies of data analysis, modelling and/or simulation aspects as this is not my area of expertise; however, I know with certainty that you would require valid and accurate data for your analysis purposes. The difficulty is that you would not have access to such valid and accurate data at least for the evaluation of tablet or capsule products for the prediction of plasma drug levels or profiles. That is, in vitro drug dissolution results which represent or simulate in vivo dissolution and by extension plasma drug levels or profiles. You would require such data to validate your simulation or modelling outcome at least for the product development and manufacturing stages. Unfortunately, no one, at present, is generating, or can generate, valid in vitro dissolution data, thus your efforts of conducting simulation/modelling are regrettably of no use and would not help the industry, regulatory authorities or anyone else. Please, do not make claims of the successes and usefulness of such exercises.
One of the main reasons for not being able to obtain valid in vitro dissolution or drug release data is that the recommended and required (e.g. from USP and FDA) dissolution testers for such purposes have never been shown to provide valid and accurate dissolution results i.e. these testers have never been validated for their intended use or purpose. Vendors/manufacturers make extraordinary efforts and take pride in providing “compliant” testers i.e. meeting or exceeding “physical or fixed” specifications according to the pharmacopeial (such as USP) requirements; however, unable to validate the testers as dissolution testers. For example, no vendor, at present, can provide you valid in vitro dissolution results if given a blinded sample of a tablet/capsule product. Therefore, in this respect claims made by the vendors are also not accurate that they are selling or manufacturing dissolution testers. At best, the only claim they can, or should, make is that they are selling simple stirrers. Perhaps more disturbing is the fact that these stirrers when used as required for dissolution evaluations, because of their design and operation limitations and flaws, cannot provide valid and accurate dissolution results which is documented extensively in the literature.
In short, please use and promote the simulation and modelling techniques with care and certainly use extra caution in making claims for such about the future expectations and successes.
Regulatory requirements for establishing quality of pharmaceutical products – serious scientific and cGMP deficiencies and flaws!
[I posted the following comments on a discussion on the AAPS Community Forum; I think visitors of this website would also find it a useful read].
Thanks for your comments in response to my post. I am not sure how should I respond because focus of your comments is more of philosophical than subject matter.
Indeed I worked with Health Canada as a research scientist to provide support and critic on the underlying scientific aspects of the assignments. Most of my laboratory work has been published and views have been known publically as well – as you noted. I have never undermined any ones’, including authorities’, hard work or practices. However, through my laboratory work and related applied experience, I did find some very disturbing misunderstandings about the use of science for quality assessment of the pharmaceutical products. The drug dissolution testing is one part of it and the second more noticeable is the claims of establishing quality of the manufactured products in particular tablet and capsule. These misunderstandings should be highlighted and addressed, in my opinion, not that be ignored and concealed otherwise everyone involved in it will lose their credibility, in particular scientific, for a long time to come.
Your statement that “We, working in the industry, have worked very hard trying to make it work.”, I am sorry what does it mean? Can you determine dissolution characteristics of a given blinded product sample? Have you used a validated dissolution tester for dissolution testing, how? How could you or anyone else develop a valid dissolution method without the availability of a validated dissolution tester? Are you able to define and establish quality of a product using drug dissolution method, how? Please, note that quality of the products is not even defined yet with a measurable parameter, then how could a dissolution test be used as a quality control tool? These are some of the unanswered questions about the use of flawed science and its practice in manufacturing and regulatory assessments. These questions are not directed towards you as a person but to the industry and regulatory authorities in general. I do not know how I can specifically direct my concerns only to the regulatory authorities. I do not think that AAPS Community Forum is only for the industry. I observed it is equally read and participated by the regulatory scientists as well. A discussion was just started on this community forum by a scientist from FDA (e.g. see under METHODS IN IMAGING DATA ANALYSIS).
So please join hands with me and inform the regulatory authorities that there are serious problems in regulatory requirements rather than suggestion of avoiding discussions of these issues. This would not be public service or service to patients but something else!
Regarding the scientific aspect you noted from my post “A dissolution method should not be used for product development until and unless it has been clearly shown …………”, this is not my view or my suggested requirement – this is general principle of science and regulatory (i.e. cGMP) requirement. If anyone is not following or meeting this requirement then the results obtained would not, at least should not, be accepted under (cGMP regulation as an example I quote the following three FDA regulations for your information [21 CFR 111.320, 21 CFR 820.72, 21 CFR 211.194 (a) (2)].Please correct me if I am wrong on this.
In the end, please consider using this forum informing the authorities that fundamental scientific principles, as well as cGMP requirements, are being violated which need to be addressed so that industry could be able to function appropriately and public should receive accurate and honest information about the quality of the manufactured pharmaceutical products.
I will be happy to provide help to anyone in explaining the issues and suggesting possible solutions to such if you suggest or provide leads in this regard. I look forward to future fruitful discussions on the subject with you.
With best regards.
Citizen Petition (FDA) – Requesting withdrawal of drug dissolution apparatuses from FDA regulatory requirements
Today, I have submitted a Citizen Petition on the FDA site on the above mentioned subject (Tracking Number 1k2-94p3-9n4b). The content of the petition may be found here. I will keep you updated with the progress).
A revised Citizen Petition has been registered with the FDA (FDA-2018-P-3742) and can be found here (https://tinyurl.com/y9oaxe5t). (October 3, 2018)
F2 (similarity factor): An arithmetic skill-test – not a widget for quality assessment of pharmaceuticals
While surfing the internet, I found a prize Claim Form from Tim Hortons (Coffee Shop), which requires the winners to complete a skill test, a simple arithmetic exercise, to claim the prize (link). The exercise goes like this: multiply 2×4, add 8, subtract 4, add 6 and then show the correct answer. The exercise is unrelated to the quality or value of the prize, but a requirement for receiving the prize.
It reminds me of F2 (similarity factor) requirement, which is a very similar arithmetic exercise as well, with added parameters of taking logarithm and square root of numbers to come up with an answer to receive the “prize” of “regulatory compliance”, i.e. regulatory approval of your product (usually tablet/capsule) as bioequivalent with or without a human bioequivalence study. The point being, the skill test, in this case “similarity factor” unrelated to the product quality (scientifically, statistically or otherwise) and/or lack relevance to human bioequivalence study, but is required to meet a compliance requirement (link).
BTW, if you would be using a scientific calculator or computer spreadsheet for the calculations, then you might also be required “validation” of the calculator and spreadsheet software, and its use, to confirm if they or you are performing proper calculations for which one might require help of a CSV (Computer Software Validation) expert or consultant.
Just a thought in case you are considering using F2 (similarity factor) for your studies or product evaluation for regulatory compliance – otherwise you do not have to worry about this factor as this is pretty much useless exercise unrelated to quality aspect of the pharmaceutical products.
Data integrity/management violations or accommodating flawed regulatory requirements for compliance?
It has almost become fashionable to talk out loud about data mismanagement and integrity issues in the pharmaceutical industry highlighting lack of trust in the industry’s competency and honesty by the regulators and associates, which is really unfortunate. Please, do not be this distrustful! Many times many influential people in regulatory agencies come from the industry and vice versa. People cannot be dishonest only on one side. I doubt that the pharmaceutical industry has a higher ratio of bad behavior to good behavior when compared to any other industry – perhaps less.
In my opinion and experience, people in the pharmaceutical industry are doing their best to accommodate flawed (non-scientific and illogical) regulatory requirements. It is the regulatory authorities which have to correct their assessment methods and approaches including inspections.
For example, considering the case of generic product assessments, both in vivo (bioequivalence) and in vitro drug release/dissolution, are based on scientifically invalid methods and/or testers which cannot provide accurate and reproducible results as well as quality products. This would simply be impossible. To bring the data/results within acceptable range to meet compliance requirements, not the quality which is undefined as of yet by the authorities, the industry has to “play” with numbers and techniques such as repeats and discarding test results. This appears “data fudging” (or “dishonesty”), most often to those who have limited expertise and experience working in the laboratory environments. Please avoid such blames under the practices of computer software validation, data integrity, data security, missing data trails, etc. These are neither helping nor addressing the underlying issues.
The only possible solution to address this lack of trust situation is that the regulatory authorities, including pharmacopeias, have to clearly define quality of the products with an objective measurable parameter. Let the industry meet these standards using scientifically valid and qualified methods not working with the flawed science imposed by the authorities.
The following are some relevant links provide further details in this regards:
http://www.drug-dissolution-testing.com/?p=3069
http://www.drug-dissolution-testing.com/?p=3065
http://www.drug-dissolution-testing.com/?p=3007
Quality of pharmaceutical products: “Regulatory Science” – its illusions, obstacles and a potential solution.
“Regulatory Science” is a term often used to describe practices of national, sometime international, bodies to establish and monitor quality of pharmaceutical products such as tablet and capsule, which would include safety and efficacy aspects as well. A clear description of the term “Regulatory Science” appears to be lacking. In practice it may be considered as a practice of setting standards (specifications) and protocols for describing and establishing quality of products available on the market for human use. The underlying concepts for setting standards/specifications and protocols usually come from the fundamental principles and laws of sciences, engineering and mathematics such as biology, chemistry, manufacturing and statistics. “Regulatory Science” uses these scientific principles to set specifications and protocols, rather than generating new scientific knowledge which is generated under the auspices of a specific scientific and/or engineering discipline. Therefore, regulatory bodies hardly ever generate new scientific knowledge but use it to generate specifications and standard procedures for implementation for the public good. The authorities also get the mandate to enforce the developed and suggested specifications and protocols – as these are intended to be followed. This mandate of enforcement results in the term “compliance” i.e. industry must adhere (“compliant”) to the standards and protocols for their manufactured products to be approved for marketing. Continue reading
Time to rescind the regulatory requirements of bioequivalence evaluations and the current pharmacopeial drug dissolution practices as these do not provide quality assessment of pharmaceutical products
Considering the non-specificity, because of confounded variabilities from the physiological system, drug release assessment of pharmaceutical products (tablet/capsule) for which this test is conducted, the bioequivalence test becomes a scientifically and statistically in-valid practice. See here for further discussion on the topic (1,2).
An in vitro drug release test, commonly known as drug dissolution test, which by its nature avoids the above mentioned non-specificity, provides a better alternative for assessing drug release characteristics of the products thus their quality. Pharmacopeias worldwide recommend this test. Unfortunately the recommended testers suffer a serious design problem thus providing irrelevant and unpredictable results not reflecting product quality or lack of it (3,4). In short, the drug dissolution tests as currently recommended are based on non-qualified and/or non-validated testers, hence results from the testing cannot be relied upon. Therefore, their use is to be discontinued as well.
As a solution, a simple revised dissolution testing approach has been suggested which would provide superior drug release evaluation thus quality of the products for human use (5, 6). In addition, as it is an in vitro technique, the test can be conducted without the use of human subjects avoiding unnecessary risk to participating healthy volunteers and/or patients. The suggested approach not only provides scientifically valid method for assessing quality of the pharmaceutical products but also would give much needed flexibility to pharmaceutical industry for innovation to bring out products faster, and with a reduced price, into the market.
Quality generic products without bioequivalence (BE) assessment – a simple and practical approach!
Considering the weakness (non-specificity) of BE assessments it is suggested that in vitro drug dissolution/release testing would provide a better alternative to establish quality of pharmaceutical products such as tablet and capsule. It is argued that the use of in vitro dissolution test should be the method of choice for developing and monitoring improved or better quality generic products because BE assessment focuses only on equivalence and not on the improvement of the product quality. Other significant advantages of using an appropriate in vitro dissolution test in lieu of BE assessment are described..
For further detailed explanation please follow the link.
Pharmaceutical products quality and bioequivalence assessments – what a waste and needless use of human subjects!
A bioequivalence study is conducted in humans to establish that two or more products are capable of providing same/similar blood/plasma drug levels. Underlying assumption is that if the products provide same plasma drug levels then their therapeutic effects would be the same as well, thus would allow interchangeability of the products such as the generics.
Therefore, for all practical purposes the bioequivalence assessment may be considered as a typical analytical chemistry test where the assessment is based on determining plasma levels. For conducting an appropriate and accurate analytical test, the test must follow some fundamental principles of analytical tests such as specificity and its validation (accuracy, precision and reproducibility). A test cannot be validated if it is not specific.
In this regard, a bioequivalence test is a non-specific test as plasma drug levels include (confounded) variabilities from stomach emptying/motility and liver metabolism of the drug – independent of the product characteristics. Therefore, caution is warranted in establishing quality of the test products based on the bio-equivalence test.
For further detailed explanation please follow the link.
Pharmaceutical product manufacturing as per current regulatory requirements!
Consumers and patients must wait, and suffer, for the availability of quality pharmaceutical products such as tablet/capsule as well as their genuine and affordable prices. The reason may surprise you!
It is important to note that at present availability of the pharmaceutical products such as tablet and capsule is heavily regulated, more accurately controlled, by the regulatory authorities worldwide. Manufacturers and suppliers have to follow extensive suites of protocols (national and/or international) to get their products approved for marketing. These protocols are often described by different names such as regulations, guidelines, standards etc. The manufacturers have to be in compliance with these protocols literally to the letter, which are mostly arbitrary in nature. Thus, in practical terms contrary to popular belief, there is limited or no room for deviation, simplification and/or innovation from these protocols at least from the manufacturers’ side.
In simple terms, these protocols may be considered as formats for data/results presentations, may these be for the product development or manufacturing – promoted as regulatory science. However, unfortunately, these are administrative and procedural requirements, not the practice and/or requirement of the science. The underlying “science” remains based on traditional practices and assumptions, more accurately may be considered as rituals. Therefore, with the passage of time and the introduction of extensive sets of standards and requirements, the burden of adhering to these regulatory formats (“guidelines”) has become increasingly frustrating, time consuming and financially challenging for the both, authorities and the manufacturers, without any added value to the product quality and/or benefit to the users.
In addressing these challenges, manufacturer bashing approaches (implied or explicit) are common and fashionable, often criticizing lack of their integrity and competencies. This approach certainly appears to be a deviation away from the regulatory mandate or requirements which is establishing and monitoring quality of the products and not that of assessing and criticizing manufacturing ability or capacity. Regulators’ and their associates should be able to establish if the manufactured products, at the consuming stage, are of the required quality, and by extension, safe and efficacious. However, they can’t at present – thus deviation from their mandated objective!
There are two reasons for this regulatory shortcoming: (1) Regulatory authorities have never defined required quality, and its associated parameter, for the product assessments. In fact, it could be argued that it is unknown to them. (2) Authorities require and enforce a large array of flawed product testing requirements for compliance purposes without their validations and relevance. As these requirements lack scientific credibility and validity, anybody, not just the manufacturers, would have difficulty in meeting or will be unable to meet the current regulatory requirements and expectations. For a more technical description of this aspect please consider viewing the links provided below.
Therefore, there is a clear need for re-evaluating the practice of setting regulatory standards and requirements starting with the definition of a quality product followed by the use of scientifically/GMP valid instruments and procedures. Otherwise, it is impossible for the manufacturers to produce quality products, and for the regulators developing and implementing appropriate guidelines and standards for product evaluation.
Some suggestions are provided to address these issues, and it is sincerely hoped that authorities will give consideration to these thoughts.
For further reading:
(1) http://www.drug-dissolution-testing.com/?p=3022
(2) http://www.drug-dissolution-testing.com/?p=3007
(3) http://www.drug-dissolution-testing.com/?p=2956
(4) http://www.drug-dissolution-testing.com/?p=3037
(5) http://www.drug-dissolution-testing.com/?p=2922
Are bioequivalence (BE) assessments of clinical significance and relevance? Not really!
A discussion is provided showing weakness of BE assessments for comparing or establishing quality of products such as tablet/capsule. It is argued that in vitro drug dissolution/release testing would provide a better alternative for the assessment of the quality of such pharmaceutical products. Please click here for complete article.
Regulatory compliance is more appropriate description than QC/QA
It is important to note that at present pharmaceutical laboratories are operating under non-GLP/GMP conditions, in particular for the assessment of solid oral dosage forms such as tablet and capsule products. This is surprising that such negligence has been going on unchecked. This deficiency needs to be corrected so that facilities can be considered as QC/QA laboratories to provide relevant and accurate quality characteristics of the products. For further details, please follow the links (1, 2, 3).
Need for a quantifiable quality parameter for pharmaceutical (tablet/capsule) products
People try to understand the logic and scientific principles behind the (pharmacopeial and regulatory) “compliance” requirements for meeting the quality aspect of the pharmaceutical products such as tablet/capsule. However, there is hardly any scientific principle involved in most of the current “compliance” requirements in the area. Most compliance requirements are based on subjective (individual or collective) opinions and guesses, often presented through publications or regulatory guidance documents to gain or establish their authenticity. For example, in the area of establishing quality of the manufactured products, such as tablet/capsule of both generic and branded products, nowhere it is defined what would be considered as a “quality product” and how the quality should be measured or established. However, all the pharmacopeial and regulatory requirements (national or international) make claims of achieving it. Is it not interesting that quality of a product is not defined or known, but claimed to be achieved, how?
Quality assessment of pharmaceutical products – regulatory/pharmacopeial standards and methods require urgent attention!
A patient needs a drug but prescribed or purchases a drug product (such as tablet or capsule) with an implied assurance that product will release the drug in the body as expected to provide its therapeutic effect. This characteristic of drug release/delivery in the body from the product defines the quality of the product.
At the manufacturing stage one hardly ever conducts clinical tests to establish the safety and efficacy of the products but only the “quality” tests. The reason being if the quality is acceptable then safety and efficacy will be acceptable as well because drug levels in the body and safety and efficacy are directly linked.
The tests most often conducted to establish quality of products at the manufacturing stage are the chemical tests, commonly known as in vitro tests. The most common in vitro test which is conducted to establish drug release or quality of the tablet/capsule products is known as a drug dissolution test. This is the only test which forms the basis of quality assessment of tablet/capsule products. Regulatory authorities, including pharmacopeias, worldwide recommend and enforce proper use of this test to establish the quality of the products. There are numerous guidance documents available from the regulatory authorities, including US FDA, which describe the requirements of the dissolution testing procedures forming the basis of drug product approvals.
The important, and perhaps a very disturbing, fact to note here is that the dissolution testers, along with associated methods, as recommended by the authorities have never been validated for their intended use or relevance. In fact, many scientific studies (e.g. link) have clearly shown that the tests do not and cannot provide relevant results i.e. the testers cannot provide accurate results/data regarding the products (tablet/capsule) quality. Therefore, any claims made regarding the quality of the approved products by anyone, including authorities, lack accuracy and scientific authenticity.
The regulatory authorities enforce extensive set of requirements and standards through elaborated set of “compliance” guideline such as ICH or US FDA guidance documents for establishing quality of the products. This guidance-based compliance system is directly or indirectly dependent on the drug dissolution test at least for tablet/capsule products. Therefore current guidance-based system is not only providing false assurance about the quality of the products but also causing severe hindrance for the industry to produce quality products appropriately and efficiently. In addition, this guidance based system adds enormous administrative burden for both, authorities and manufacturers – unfortunately of no or limited benefit to either party. Furthermore, as the recommended testers are non-qualified/non-validated which makes them non-GMP compliant, thus authorities could easily be found in violation of GMP practices – potentially a serious and disturbing claim. Therefore, this deficiency should be addressed on an urgent basis with the highest priority.
It is important to note that scientific studies have clearly shown that currently recommended apparatuses have a design problem which could be corrected by simple modification. Suggestions have been made in the literature in this respect addressing the issues. Further information in this regard may be obtained from here.
In short, authorities should take a note of this critical deficiency which exists in the drug product approval requirements caused by requiring non-GMP drug dissolution testers. This deficiency needs to be addressed quickly so that quality of the manufactured products could be established and monitored accurately and efficiently.
Quality equals safety/efficacy of the pharmaceutical products
[This post results from a query to one of my posts on LinkedIn Network (link), I think visitors of my website would also find it a useful read]
I believe your view/confusion is valid and understandable considering the current state of drug product evaluation practices. Note that this confusion occurs from considering or mixing drugs and drug products as the same. You are not alone with this confusion; even the regulatory authorities are confused with it, in fact indirectly extending it. For example, for generic product evaluations US FDA requires ANDA (abbreviated new DRUG application, in Canada ANDS), which is an incorrect terminology and in reality these are new PRODUCT applications (link). Your confusion also appears to be arising from the same mix-up i.e., not differentiating the drugs from drug products.
My post is regarding PRODUCTs, where one hardly ever conducts clinical tests/studies especially at the commercial production stage. Safety and efficacy studies/evaluations of DRUGS are done in the beginning, sometimes decades ago (e.g., consider the examples of aspirin, acetaminophen, ibuprofen, and others perhaps most drugs). From these clinical studies dose levels are set based on their safety and efficacy profiles.
At the production stage these drugs as PRODUCTS are manufactured worldwide without any further clinical evaluation. At the production/manufacturing stage objective is to produce PRODUCTs of the drugs which must contain the required dose and they must also be capable of providing (often described with terminologies of releasing/delivering/bioavailability) expected amount of the drug in the human body whichever route of drug administration is suggested (often time it is oral, but could be others IV, dermal, sublingual etc.). There would not be any concern or focus here for the safety and efficacy of the drug, along with other non-actives or excipients, which have been already established. In the generics cases, these are assessed often by bioavailability or bioequivalence assessments which is also usually one time shot and often at the pre-production stage. The point being that at the production stage there is hardly any (clinical) safety and efficacy assessment.
On the other hand, at the production stage safety and efficacy refer only to the ability of the product to release drug/dose as expected and this becomes quality metric for the product. If a product does not deliver/release the drug/dose as expected it would not be of quality which mean (equal to) it would not be efficacious and/or safe. This is where equality comes from (you may consider it as mathematical relationship if you like which may not be incorrect either), however, we are dealing with yes/no, pass/fail, or equal/not-equal situation.
So, in short, at the production stage of the PRODUCTs, quality is equal to safety and efficacy.
Compliance/guidance-based regulatory system and products safety and efficacy assessment
Safety and efficacy of the pharmaceutical products (e.g. tablet/capsule) can only be established by determining quality of the products. If quality of the products cannot be established, as currently is the case, then claims of safety and efficacy cannot be made either. In addition, as compliance does not necessarily equate quality, safety and efficacy cannot be achieved by compliance as well. Use caution in promoting or accepting such claims. Quality of the products, and by extension their safety and efficacy, has to be defined and determined independently.
Please seek definition of the quality of the pharmaceutical products in terms of a quantifiable parameter to substantiate claims of safety and efficacy. For further details the following links would be useful (1, 2, 3) .
Validation – what it really means!
It is really hard to believe or accept how people have been blind-sided with “validation” (re-validation, continued validation etc.) requirement. The simple fact is that to run validation for any process one requires a reference product or parameter to show that process is validated (or capable of providing an expected outcome or product). If one does not have a reference product with known parameter and its value, then it is impossible to validate anything. Please, people this is logic and science 101.
If anyone is requiring or conducting validation without a reference (as in the case of current practices of manufacturing of quality pharmaceutical products in particular tablet/capsule), then validation of such manufacturing processes must be considered as “abracadabra” practices commonly accepted as meeting the “compliance”, “harmonized standard” etc. and do not link to quality aspect of the process or product.
I hope authorities, including pharmacopeias, will work in addressing these bizarre trends of validation approaches/concepts currently in practice and/or required, which have no scientific and/or logical basis.
Please, define a quality product and then provide a reference product which would allow the manufacturers to meet or exceed the standards of quality.