KABOOM! Pharmaceutical Product Quality Issue
A quality pharmaceutical product – please define it?
Something to think about!
Drug Dissolution Testers (Paddle/Basket)
Availability of pharmacopoeial reference standards for medicinal products?
Vendors and Dissolution Testers
Year End Thought on Quality of Pharmaceuticals
My recent presenatation
Last month I gave a seminar in Lahore, Pakistan organized by the Pakistan Pharmaceutical Manufacturers’ Association (PPMA). A copy of the presentation (slides) is attached (link). If you have any comments and/or questions, please do not hesitate to contact/write me at moderator@drug-dissolution-testing.com
Some suggested rationales for using a non-compendial dissolution tester and/or method
There are a number of reasons where one can use, in fact should use, non-compendial testers and methods, e.g. when currently recommended compendial testers/methods are:
- Unable to determine dissolution characteristics of a blinded sample, a common and standard practice/requirement for any analytical test.
- The usefulness (validity) of the tester/method is in question i.e. a tester/method is not capable of determining and differentiating dissolution characteristics of products. For example, they are unable to differentiate between immediate- and extended-release products having the same active ingredient.
- The underlying science is weak or invalid e.g. the test method is product dependent. It is a serious scientific violation that the method used is product dependent.
- Complex and time consuming.
At present, pharmacopeial testers/methods are deficient in all the above mentioned characteristics/requirements. Therefore, if a newer approach addresses any or all of the above mentioned deficiencies then automatically the new or modified tester/method becomes superior and should be used. People should focus on addressing and improving on the current methods and pro-actively suggest newer methods to the agencies. Analysts/scientists should give no consideration to views that only pharmacopeial testers/methods should be employed to avoid potential delays in product approval. It is much better to spend some extra time up front to have a scientifically valid and robust method than using the recommended method/approach which is flawed as it will cost a lot more during production cycles, failures (OOS), recall, etc.
It is important to note that modified testers/methods should be suggested at the stage of product application, where it would be relatively easier to be accepted. It is highly unlikely to change at the production or plant inspection stage, as specifications are part of the “contract” which are very difficult, or impossible, to change.
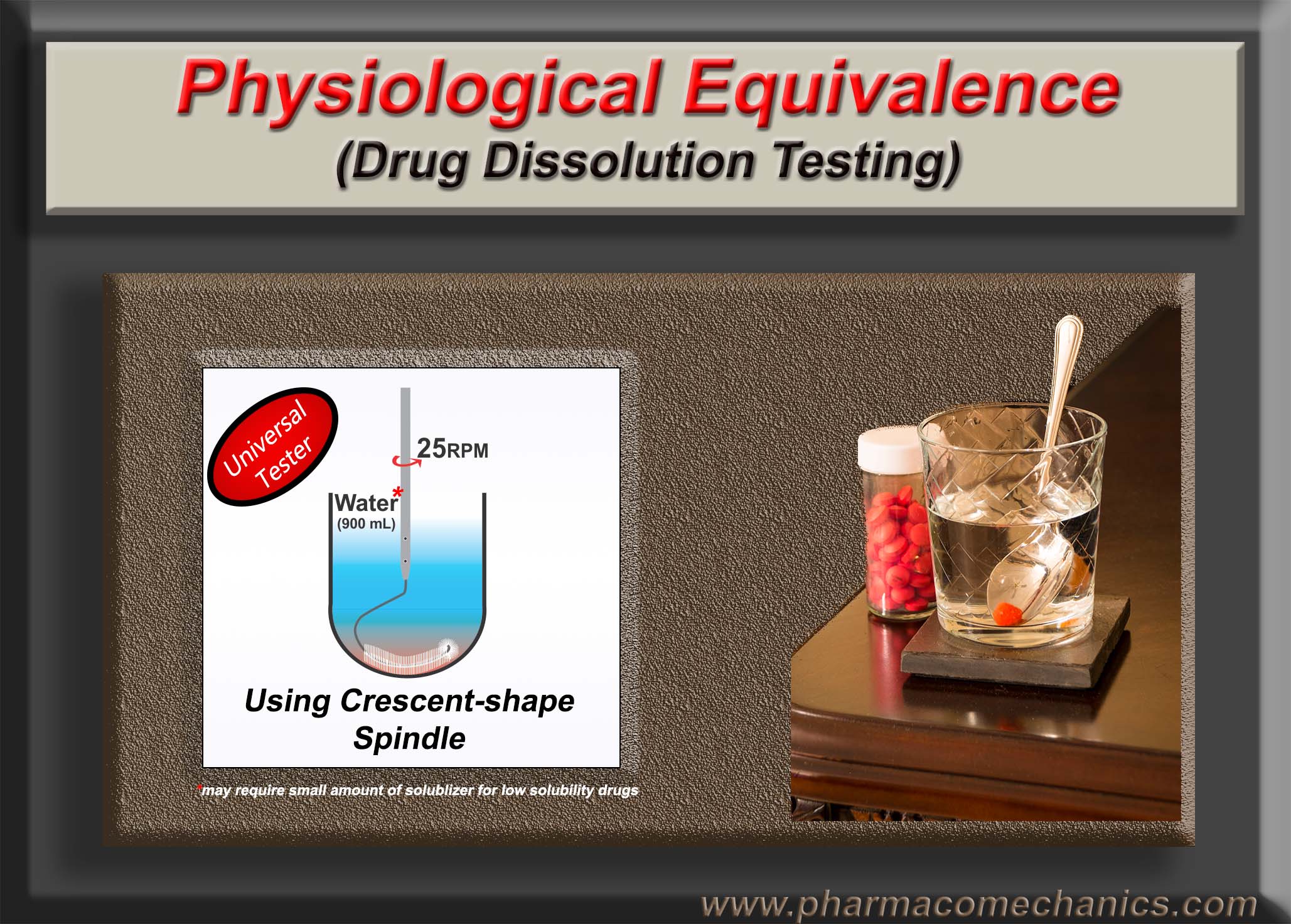
The Crescent-shape spindle (link) has been suggested for drug dissolution testing to address the deficiencies of the currently recommended apparatuses and methods. In addition, its use significantly reduces work-load, thus increasing efficiency and cost savings.
Comparing Quality Standards – Pharmaceutical vs Consumer Products
The quality of pharmaceutical products such as tablets/capsules may be defined as their ability to provide or release the drug present in the product in humans in an expected manner. It is important to note that the need of a consumer or patient to take a drug product is to have the drug (active ingredient) delivered to him/her in the right amount and in an expected manner. A product provides only the mean to deliver this drug. Continue reading
Bio-waivers!
Please consider the basis and do not make up the science!
A simple and practical approach for establishing credibility and authenticity of the help and advice provided in the practice of drug dissolution testing
Drug dissolution testing is a well-established analytical technique or test, and is extensively employed for the development and assessment of pharmaceutical products in particular the tablet and capsule. In fact, it would not be an exaggeration in saying that it is the only test which is used for establishing the quality of products. However, it is also a fact that the tests and testers as recommended have never been qualified and validated for their intended purpose. Therefore, tests and testers cannot provide relevant and scientifically valid results regarding the quality of the tested products. This is simply common sense and scientific fact.
Some however, still promote the current practices, through trainings (conference, seminars, and write-ups) as scientific and useful which are not only causing delays and hindrance in addressing the issues at hand, but also create false hope for analysts/scientists that this technique may provide useful and relevant results. Sometimes this training and advice is disguised in different fanciful names or topics such as clinical or bio-relevant, IVIVC, bioavailability/bioequivalence assessment, but underneath it all are flawed dissolution results. Reality remains that it is almost impossible to obtain reproducible and relevant dissolution results for any product using the currently suggested dissolution testers.
A simple and practical approach one could use to assess the credibility or authenticity of promoted claims and expertise is to request if the person (trainer or vendor’s representative) is able to determine dissolution characteristics of a given blinded sample of a product containing a highly soluble drug using an independently developed and validated method (see below). If yes, then certainly it could be a good source for learning, otherwise, one should use caution.
Please use caution and pay attention (link)
Quality assessment and prevailing illusions!
The main promoted claim from regulatory authorities such as the US FDA, Health Canada and other national and international agencies concerning pharmaceutical products, such as tablet/capsule, is that they ensure that the public or patients receive quality medicinal or drug products. In this regard, it should go without saying that to establish quality of products in a scientific manner, one would require to define the quality of a product in an objective manner with a measurable metric. However, at present, regulatory authorities worldwide do not provide such a definition or metric (link). Therefore, it is not possible to establish and/or assess the quality of the manufactured products.
Current practices of proclaimed monitoring and/or establishing the quality of products are based on traditional views and opinions compiled as Guidance documents and related compliance requirements. Although such documents and requirements are numerous of increasing complexities, their content remains subjective and arbitrary in nature, and in many cases scientifically invalid and contradictory. This has given birth to fantastic illusionary practices of quality assessment named as pharmaceutical science, regulatory science, risk management (science), quality management (science), compliance, inspections, etc. However, the fact remains that no one is monitoring the quality of products nor are they able to monitor it, as the “quality” is an unknown or undefined entity. This is a fanciful example of an “illusionary science” promoted and practiced by most “experts” in the area causing delays and hindrances in obtaining and manufacturing of products in a cost effective and timely manner.
Such an impeding, confusing and illusionary situation can be eliminated, if one would work with defining the quality of a pharmaceutical product which by itself is relatively easy to define as suggested here (link). It is therefore humbly requested that regulatory authorities take note of this request and consider including a definition of a quality product so that needed quality products could be manufactured in a timely and efficient manner.
Quality by Science – A Suggestion for Change!
Link for relevant discussion (1)
Response to a recent query: Scientifically all dissolution results obtained using currently recommended apparatuses would be null and void and non-GMP compliant!
Query:
Hello sir.. How r U? I want to know about Dissolution. If my product (Solid oral dosage form) is not available in any pharmacopoeia for dissolution than how can I select dissolution media, apparatus, RPM etc.??? how to develop dissolution method? its depend on solubility of drug or absorption of drug? Please sir help me about this topic. Thank you,
My response:
Thanks for asking the question and your interest in my expertise. Please note that as it stands, considering what is available in literature including from US Pharmacopeia (e.g. General Chapters <711> and <1092>) and available guidance documents such as (from FDA, Health Canada and Europe), it is NOT possible to develop a dissolution method or select relevant experimental conditions to conduct a dissolution test. People do promote such practices, however, the fact remains that one cannot determine dissolution characteristics of any product using the currently suggested apparatuses and method. One of the main reasons to this effect is that currently suggested dissolution testers are not qualified/validated for dissolution testing purposes (e.g. see here). Therefore, any results obtained using such testers, in particular paddle/basket, would be null and void and GMP non-compliant.
On the other hand, considering the limitations of the current practices, I have proposed a slightly modified dissolution tester using a new stirring element known as Crescent-shape spindle, which provides a simpler and scientifically valid approach for conducting dissolution tests (1, 2). In this case, you would not need to develop a dissolution method at all, and dissolution tests can be conducted using a very simple set of experimental conditions. If you require more details in using Crescent-shape spindle, let me know, I will be happy to help.
Best of luck!
Shifting Focus – Update
Seriously, it is not science. Please, stop promoting it as science!
Link for relevant discussion (1)
Inspections and quality of pharmaceutical products and/or manufacturing processes – dilemma!
Link for relevant discussion (1)
A recent query!
Question:
How i can enhance Pioglitazone Hydrochloride dissolution profile results in combination with metformin HCl tablet ????
My response:
It is a common question/issue i.e. people ask that they do not observe expected or desired drug dissolution characteristics of a product, but faster or slower. The simple and direct answer to such a question should be to change/adjust the product formulation/manufacturing attributes accordingly. In fact, that should be the only option or advice provided. Often as one does not know the formulation (usually not disclosed), thus it is impossible for anyone to provide any useful advice in this regard.
On the other hand, at present, formulators have been convinced and/or trained to seek changes or adjustments in the dissolution method itself to obtain the characteristics they like to see. Such a practice of dissolution method adjustments are commonly promoted as science and method development practices. I call this as “Fashionable Nonsense”, as this practice is neither scientifically valid nor of any practical use (link). So please avoid such a practice.
To address such a problem/question, I would ask how you would know that your dissolution observations/results are correct. Is your method capable of providing accurate and/or valid dissolution results? That is, is the method you used has been validated to determine dissolution characteristics of a product? The answer is NO, i.e., none of the testers/methods, as recommended at present, are qualified and/or validated for dissolution testing. Thus, one cannot determine dissolution characteristics of any product (link). The dissolution results what you are seeing are simply an illusion, not actual or true dissolution characteristics of the product. Therefore, it is not possible to address the problem using currently recommended testers/methods. This is hard to believe but it is a scientific fact.
Considering this background, I have suggested a modified stirrer, known as crescent-shape spindle, based tester which addresses the issues and provide far better dissolution testing and product characterization. You may read the details about it here. If you require further information, I will be happy explain it further.
Best of luck!
Scientifically speaking!
Link for relevant discussion (1)
Fashionable Nonsense
Drug Dissolution Testing – A Query
You may send your response to principal@pharmacomechanics.com
Using techniques, such as IVIVC, PBPK, and/or other sophisticated statistical analyses, to show validation of dissolution testers and/or their ability to predict in vivo performance is a false science and of no practical use
It is often argued that validation of dissolution tests/testers can be, or have been, achieved using IVIVC based on convolution/deconvolution (CON/DECON) methods, and more recently applying PBPK (Physiologically Based Pharmacokinetic) modelling/simulation techniques. Unfortunately, this is scientifically incorrect and an invalid view and practice.
In reality, CON/DECON and PBPK techniques are mathematical/statistical techniques which use in vitro and in vivo results obtained experimentally. While applying the mathematical techniques, mathematicians/statisticians make a fundamental assumption that the data/results provided to them are relevant and valid, obviously obtained using qualified and/or validated tests and testers. It is the analytical laboratory which ensures that the results were obtained using qualified and validated tests and testers. The important thing to note here is that CON/DECON and PBPK are applications, mostly software, used to transform in vitro results into in vivo. They are not to show or prove that testers/tests used were validated or qualified, but work on the assumption that testers/tests were appropriately validated. Stating that CON/DECON or PBPK techniques establish that dissolution results were obtained using validated/qualified tests/testers is scientifically invalid view and unfortunately reflects misunderstood concept of the mathematical/statistical techniques applied.
It is of utmost importance to note that before using dissolution results for any purpose – eyeballing, similarity factor, tolerances setting, QC/QA, CON/DECON, PBPK or any other sophisticated statistical analyses – one has to establish that results obtained were from qualified or validated tests/testers. This qualification/validation has to be done PRIOR and INDEPENDENTLY, and not using products under development or assessment. If dissolution testers provide irrelevant and erratic results, which they do especially paddle and basket, then any modelling or statistical technique will not help in getting relevant results or correcting the problem. It will be complete waste of time and resources. One must first focus on developing and using qualified and validated dissolution testers.
In short, as the currently recommended testers have never been qualified and validated independently, thus use of techniques mentioned such as CON/DECON, PBPK, simulation/modelling, and other statistical analyses, becomes void and are of no practical use. Therefore, one must first focus on establishing or developing qualified and validated dissolution testers, if valid and successful use of IVIVC or PBPK is desired
Validation of dissolution results and/or testers with exotic data manipulation exercises!
Following is response to a question/concern I posted on LinkedIn Forum. I hope you as visitor to this blog may find it useful as well. Saeed
Question:
You are always asking for references – so here is a recent example linking in vitro to in vivo that was accepted by the regulatory agencies. If the testers are not validated and non qualifed then this work would not work – but it does. Hence your arguments are nonsense as I can link in vitro to PK using the testers plus PBPK modelling. http://pubs.acs.org/doi/abs/10.1021/acs.molpharmaceut.6b00497
My response:
James: I am sorry to say that you are not following the question/concern or the science of dissolution testing. You are just trying to be bullying. Let me explain: (my comments are based on the content of the abstract, as I do not have access to complete paper. I am assuming the tests were conducted using paddle apparatus. If you desire a detailed analysis or response then please provide me a copy of the publication. Furthermore, I do see some specific flaws in the interpretation/conclusions; however, my comments are of general nature describing publications such as the one you cited.)
What is described in the publication is a typical example of matching in vitro and in vivo outcomes, which are not that uncommon in literature. Note I am using the word “matching” not correlation or validation. What people are doing and fooling others, including you, is calling “matching” and “association” of results as “correlation” or “prediction”, which is incorrect and unfortunate. There is no need to have such results from a dissolution tester, one can use anything from lab stirring bar to kitchen food processor to obtain some dissolution results (of course by adjusting experimental conditions) and match them with any of the outcomes, maybe drug levels in blood, air, space or Mars. I am almost certain that you would not follow/understand the mathematical “rituals” people use to come up with expected or desired results to impress others like you and peers. I am not impressed.
To address the question/concern, to obtain some relevancy or predictability of exercise, one has to start with a question, is the dissolution tester used to generate dissolution results is capable of providing “relevant” and “reproducible” results. Once, it is established, and then one has to ask the question, is the tester, after showing the capability relevancy and reproducibility, capable of providing relevant “in vivo” dissolution results. That will be called the “qualification” and “validation” of the dissolution testers. Now you should take this data and start relating to blood/plasma levels, using again scientifically and mathematically valid models (note that even the mathematical modelling will require its own independent validation before applying for such correlation or prediction). I will be happy to discuss this in further details if so desired. There is no qualification or validation is available here, in particular for dissolution testing, so the question/concern remains.
In short, anyone can obtain some numbers and mix them with mathematical equations to get any results one wants or likes. However, to be valid, they require prior validation, which is missing, in particular for dissolution testers and results. No question about that. You, and others, like to call this exercise modelling, simulation and validation and you are free to call anything you like. To me, it is just jugglery with numbers, thus has no scientific merit, credibility or use. Sorry!