Please Pay Attention!
For further discussions on the topic, please see the links (1) http://www.drug-dissolution-testing.com/?p=2281 (2) http://www.drug-dissolution-testing.com/?p=2288 (3) http://www.drug-dissolution-testing.com/?p=2268
A question worth asking …
For details, please see here
Dissolution Testing?
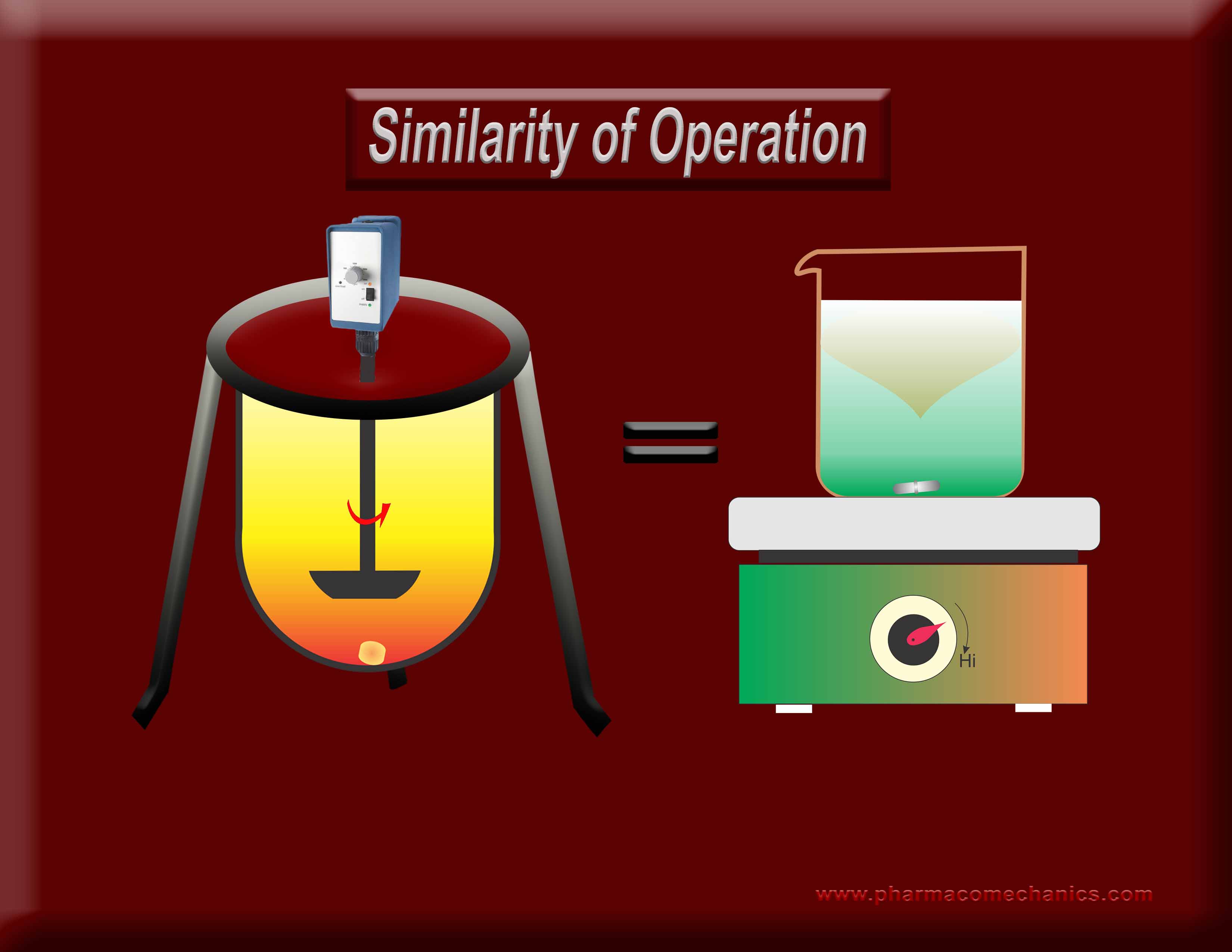
Why does one require an exhaustive standardization (calibration/PVT/Guidances/training etc.) for the operation of one stirrer type but not for the other, when both are used extensively for the same purpose (dissolution)? Does it indicate misunderstanding of the underlying concept? It appears so! To know more about as to why, both of these stirrers are not suitable for DRUG dissolution testing? See here
USP Dissolution Performance Verification Test (PVT) limits Changed (again!). Apparently retrospectively!
Focus on assessing (monitoring) quality of pharmaceutical products – disappearing?
For further discussion, please see the following links (1) http://www.drug-dissolution-testing.com/?p=2359 (2) http://www.drug-dissolution-testing.com/?p=2351 (3) http://www.drug-dissolution-testing.com/?p=2389</a>
Drug Dissolution Testing – Basic Principles & Practices
Attached is a copy of a presentation (slides) given last month at the Rowan University (New Jersey, United States) as a guest speaker for an online course (link).
is a copy of a presentation (slides) given last month at the Rowan University (New Jersey, United States) as a guest speaker for an online course (link).
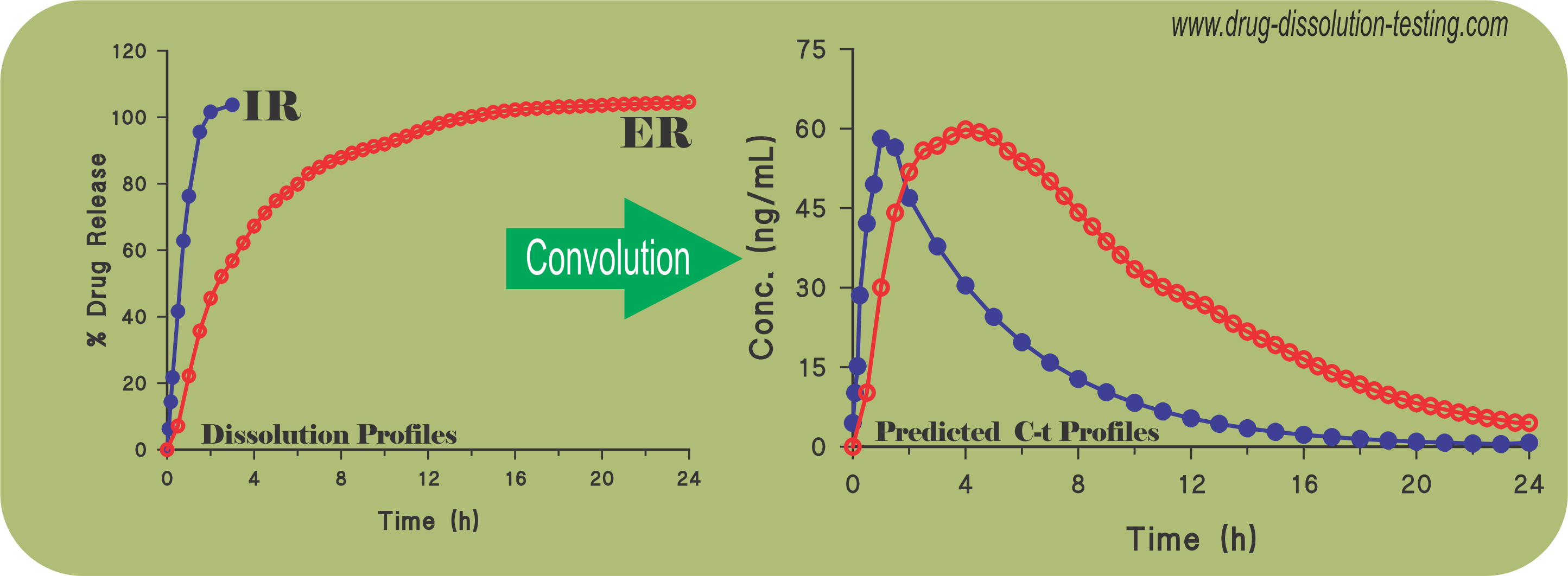
Application (validation) of convolution technique using spreadsheet software for predicting plasma drug levels from dissolution results – some examples
Recently a simple and practical approach has been suggested to predict plasma drug concentration-time profiles from drug dissolution results (link) without conducting a concurrent bio-study. The following citations describe application and usefulness of such an approach as reported in recent literature (refereed journals) from third-party laboratories.
- Solid self-emulsified nanostructures of Lercanidipine hydrochloride: A potential approach to improve the fraction of the dose absorbed. Journal of Drug Delivery Science and Technology, November 2015 (Link)
- Sunitinib-eluting beads for chemoembolization: Methods for in vitro evaluation of drug release (link).
- In vitro dissolution similarity factor (f2) and in vivo bioequivalence criteria, how and when do they match? Using a BCS class II drug as a simulation example(link).
- Establishment of a Bioequivalence-Indicating Dissolution Specification for Candesartan Cilexetil Tablets Using a Convolution Model (link).
- Study the effect of formulation variables on drug release from hydrophilic matrix tablets of milnacipran and prediction of in-vivo plasma profile (link).
- Prediction of in vivo plasma concentration–time profile from in vitro release data of designed formulations of milnacipran using numerical convolution method (link)
- In vitro to in vivo profiling: an easy idea for biowaiver study (link).
- Metoprolol-Eudragit Microcapsules: Pharmacokinetic Study using Convolution Approach (link)
- Dose regimen of para-aminosalicylic acid gastro-resistant formulation (PAS-GR) in multidrug-resistant tuberculosis (link)
- Preparation of acetaminophen capsules containing beads prepared by hot-melt direct blend coating (link)
Some related articles from this blog:
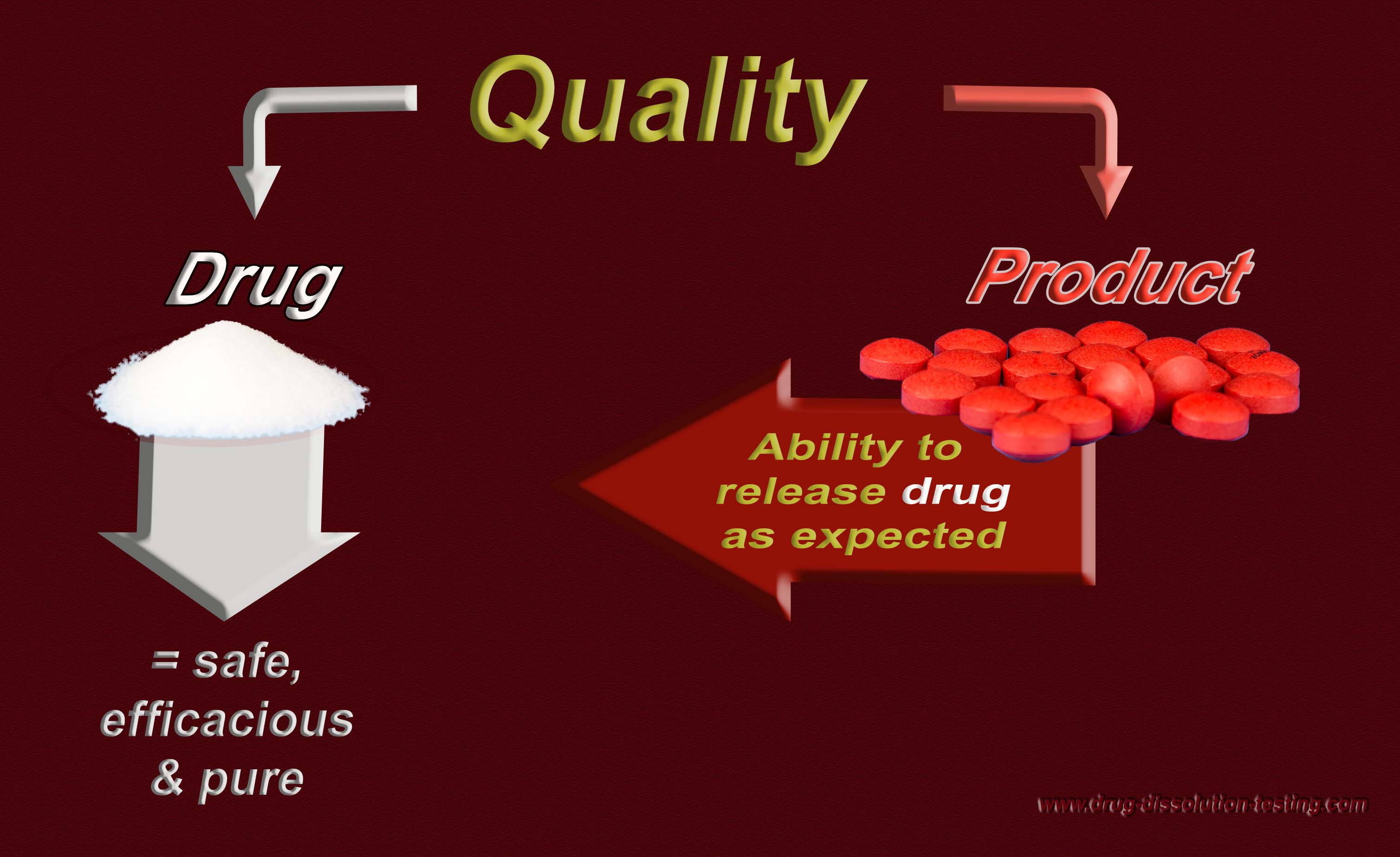
Defining/establishing quality of a drug PRODUCT – the following maybe helpful!
For further discussion, please see the following links (1) http://www.drug-dissolution-testing.com/?p=2359 (2) http://www.drug-dissolution-testing.com/?p=2351 (3) http://www.drug-dissolution-testing.com/?p=2389
Drug Dissolution Testing For Global Bioequivalence Requirements – A Presentation!
Slides from a presentation made at the 2nd Annual Bioequivalence Conference: Intersection between Science and Regulatory Summit, Philadelphia, PA, USA. September 29-30, 2015 link.
IVIVC – Calculating drug levels from dissolution results
If one likes that a manufactured drug product should be of quality, then one needs to define (tell) what a quality product is?
For further discussion, please see the following links (1) http://www.drug-dissolution-testing.com/?p=2359 (2) http://www.drug-dissolution-testing.com/?p=2351 (3) http://www.drug-dissolution-testing.com/?p=2389
Some thoughts on the recently suggested FDA Dissolution Testing Guidance
Here Guidance means: Drugs Guidance for Industry – Dissolution Testing and Specification Criteria for Immediate-Release Solid Oral Dosage Forms Containing Biopharmaceutics Classification System Class 1 and 3 (July 2015, Link)
It is difficult to clearly recognise the target audience for the Guidance. However, considering the description “This guidance establishes standard dissolution methodology and specifications that are appropriate for BCS class 1 and class 3 drugs in IR dosage form”, it may be assumed that its target audience is the analytical section/department of a manufacturer or manufacturing facility. So, validity and applicability of the Guidance should only be evaluated considering principles of analytical chemistry. In this regard, the following comments may be considered.
Presumably the objective is to establish a standard methodology; however, suggestions are of multiples. As the in vivo link or relevance of testing is not the focus, then it would be more practical and simpler to just have one set of experimental conditions: e.g. 500 mL of medium (0.01 M HCl or 250 mL which is considered more appropriate as per the guidance) using paddle method set at 75 rpm only with a tolerance of 15 minutes. This will meet the objective of “standard dissolution methodology” better for the Guidance.
From an analytical chemistry perspective, it is important to describe the fact clearly to avoid current misunderstanding that the suggested tester (paddle) is not a validated tester (not GMP compliant) to reflect dissolution characteristics of the products for human use. Therefore, the suggestion/recommendation described should be considered as a regulatory compliance requirement only, and the results/data obtained should not be extrapolated to reflect products’ in vivo behaviour and/or quality attributes for humans.
In-compliance with regulatory standards and requirements do not necessarily mean that a pharmaceutical product (tablet/capsule) or process is of quality!
It is a well-established understanding that the drug/pharmaceutical industry is a highly regulated industry. The purpose of the elaborate regulations and their implementation is that the products manufactured should be of the highest quality standards possible. There are numerous sources of such regulations, most often country- or territory-specific such as US, Japan, European etc., or some others harmonized such as from ICH. However, in general the most commonly referred, or quoted, are those from the US FDA, US Pharmacopeia, EMA and ICH.
Regulatory authorities such as US FDA, Health Canada, EMA and many others enforce such regulations and standards to ascertain that manufacturers and manufactured products are in-compliance with regulations leading to manufacturing of quality products. It is very important to note that a fundamental underlying assumption here is that if a product or process is in-compliance then the product or process will be of quality. In general such an underlying assumption is correct; however, for the pharmaceutical industry this underlying assumption does not appear to be valid. Continue reading
Current requirements of bio-waivers lack scientific merit!
Bio-waiver is a term used for product approvals, in particular tablet/capsule, without requiring an in vivo (bio-equivalence/bio-availability) testing commonly required to establish safety, efficacy and quality of drug products. For bio-waivers, requirement of bio-equivalence/bio-availability studies is substituted with in vitro drug release testing commonly known as drug dissolution testing. It is, therefore, extremely important to note that drug dissolution testing is used as a surrogate of in vivo (bio-equivalence/bio-availability) testing. Stating otherwise is clearly not an accurate view or representation of the science.
In addition, regulatory, including pharmacopeial, requirements of in vitro drug dissolution testing are based on an assumption that if products would meet the dissolution test criteria, then products would be considered as safe, efficacious and of quality for human use. This is the reason that dissolution tests are often promoted as quality control/assurance tests for pharmaceutical products (e.g. tablets/capsules).
However, unfortunately, the dissolution tests (methods/testers) often recommended and used have never been qualified and/or validated for their intended purpose. The assessment of quality of such products, using the recommended testers/methods, lack scientific support and merit. Therefore, current practices of requiring bio-waivers lack scientific merit or credibility thus their requirements should be reconsidered.
USP Performance Verification Test/Tablets – More Questions!
As per a recent follow-up note from USP (link), it is stated that “It is known that the dissolution of USP Prednisone Tablets RS changes over time.” Is this a good thing?
In principle, if the tablets, or anything else, are not stable then by default these should not be used as a REFERENCE. How would their use as a REFERENCE be justified?
On the other hand, the new validity/expiry date has been extended for six months to December 31th, 2015. Will the characteristics of the tablets change gradually or will they change abruptly on December 31, 2015? If the changes in dissolution characteristics are gradual, then shouldn’t the Ranges be changed more frequently such as weekly, bi-weekly, or monthly etc.?
Some thoughts for consideration!
Miseries of the USP Performance Verification Test (PVT)
Last week USP once again in a “surprise” announcement informed that their current lot of Prednisone Tablets for PVT is not performing as expected, so its use is halted, at least for a while. The question is, is this really a surprise or unusual occurrence? Anyone involved in drug dissolution testing, in particular PVT, would know that such occurrences are not unusual or new, but becoming relatively frequent.
Anticipation is that USP might provide some information concerning the problem and hopefully the cause of such problems. However, past experiences show that such information may not be forthcoming; perhaps, some modifications to data presentation format and/or slight adjustments in Acceptance Ranges would be made with a claim that “all is well and good to go”.
Indeed, just today (a few days later from the previous announcement) it appears that the USP has released a continuation lot, with revised and adjusted Acceptance Ranges. Such scientifically poor practices from a reputed standard setting organization such as USP are quite frustrating. USP should perhaps re-evaluate its practice of adjusting or re-adjusting the data “on the go”. Otherwise, manufacturers of the products should also be allowed to revise tolerances for their products, if their batches start giving different, or unexpected, sets of data!
On the other hand, the fact is that problems of PVT remain, i.e. frequent observance of PVT results falling outside the expected ranges reflecting high variability and unpredictability of the test. This high variability and lack of predictability in dissolution results are because of the testers (paddle and basket), however, unfortunately, USP Prednisone Tablets get the blame.
It is very important to note that to-date there has not been a single piece of evidence presented showing that indeed failure of PVT is because of the Prednisone Tablets. On the other hand, there are many scientific studies published showing that the high variability (or failures) is related to the dissolution testers (paddle and basket). Performance testing using Prednisone Tablets merely reflects the variability of the dissolution testers. It is just like if one’s computer monitor blacks-out or flickers often, one has to assess separately whether the problem is associated with the monitor or the computer. If the computer is causing the problem then adjusting or changing the monitor would not help. Similarly, in case of dissolution testing, adjusting or changing the Performance Tablets, and/or their Acceptance Ranges, would not help. USP must demonstrate that dissolution testers (paddle and basket) evaluated separately and independently from the Prednisone Tablets are capable of providing predictable and relevant results i.e. these testers are validated and qualified to evaluate dissolution characteristics of something or anything. Once this validation and qualification step has been accomplished then these testers should be used for establishing performance using Prednisone Tablets and/or actual drug products.
It’s hoped that the USP will use this latest miserable situation to address the well-known problem of the dissolution testers, not just by re-adjusting the Acceptance Ranges. Please consider providing a validated and qualified dissolution tester.
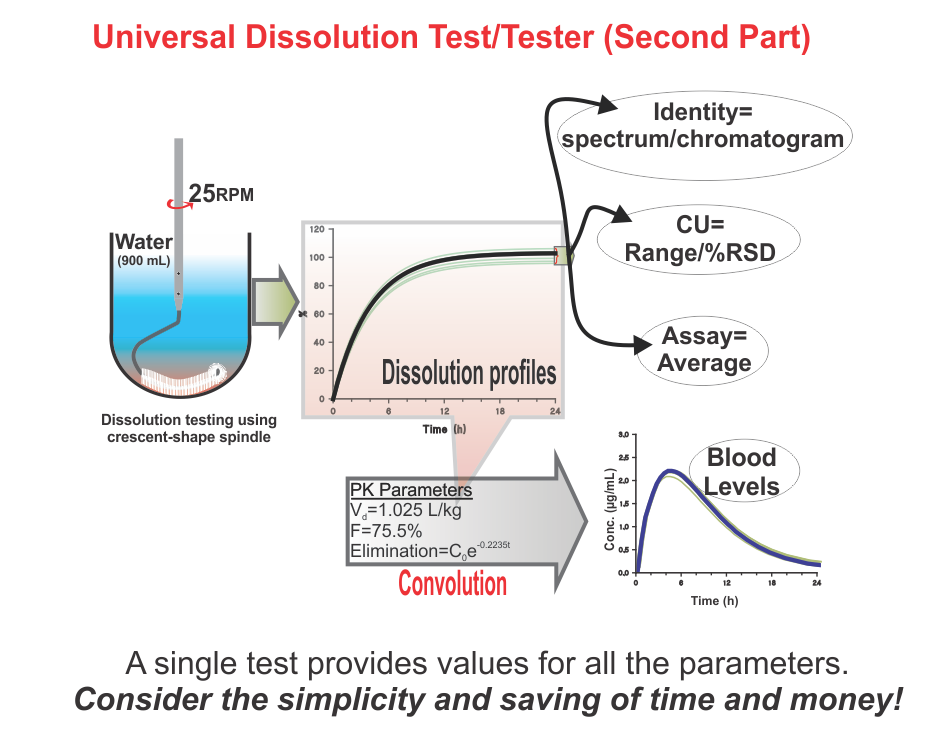
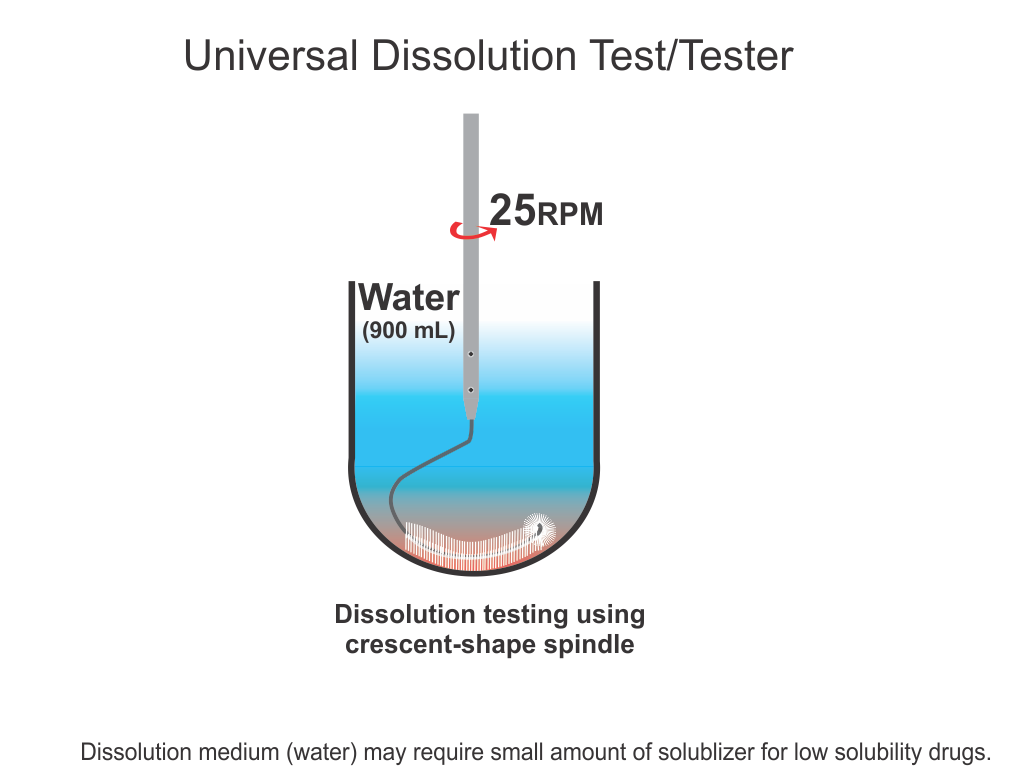
Universal Dissolution Test/Tester – Second Part
Universal Dissolution Test/Tester
Promoting quality standards for drug products: Scientifically speaking, please be systematic and logical!
It appears that talking about the quality of drugs/pharmaceuticals and their products has become a fashionable topic, in particular with respected scientists and regulators. There appears to be clear fearmongering and a blame-game approach that is taking place to discuss the subject and perhaps to gain personal or professional recognition along the way. Such human elements are natural or expected; however, when it comes to science then such emotional distractions should be managed, preferably set aside. This article is an attempt to provide a rationale and scientific point of view to highlight current difficulties in setting standards for developing and manufacturing quality drug-products, in particular tablet and capsule.
Please click here for the article
Establishing safety, efficacy and quality of drugs and drug-products (tablet/capsule) – serious confusion!
These terms (safety, efficacy and quality) are frequently used in literature, apparently without clear description and relevance. The lack of clarity and relevant use of the terminologies appear not only to cause confusion, but also at present seriously hinders the development and assessment of pharmaceutical products, such as tablets and capsules. It is highly unlikely that improvements in manufacturing practices of pharmaceuticals and their assessment, including associated regulatory standards and assessments, are possible without clearly explaining and objectively defining these terms. The purpose of this article is to help in explaining these terms considering the underlying scientific concepts in order to facilitate improved product development and assessment.
Please click here for the article
A big thank-you:
To all those who during the last few days wished me well, on LinkedIn and in person, for completion of my 30 years of service with Health Canada and retirement, and now initiating a new professional journey. I greatly appreciate your well wishes and the support provided during the past few years. I am fully committed to continue providing such contributions in future. I look forward to working with you in providing scientific ideas and solutions to problems and help bringing the quality products into the market faster and in a cost effective manner.
To enhance more open scientific interactions, I am initiating a LinkedIn group (rDissolution) complementary to my blog (www.drug-dissolution-testing.com), as often people requested a more open medium of interaction for my articles and blog postings which is not available on the blog itself. Please, join the forum (rDissolution) for more active discussions to learn and contribute.
I look forward to an exciting future and if I could be of any assistance, please do not hesitate to contact me at principal@pharmacomechanics.com or moderator@drug-dissolution-testing.com.
Please note the change!
As of May 29th, 2015, after 30 years of service as research scientist at Health Canada, I will be retiring. I look forward to a new phase in my career with great excitement. If I could be of any assistance, please do not hesitate to contact me by email: moderator@drug-dissolution-testing.com; Tel: +1 613-797-9815 1 613-797-9815![]() 1 613-797-9815 (cell)
1 613-797-9815 (cell)
Sincerely
Saeed A. Qureshi, Ph.D.
If different types of dissolution testers provide different results for the same product then by definition these are NOT dissolution testers but something else, because …
… a product can have only one value for a parameter at any given time. It is not possible that the same product can have multiple values or characteristics of a parameter (drug release or dissolution) at the same time.
This is just like a tablet or capsule can only have a single weight at any given time, no matter which type of balance one would use to measure it. Or just like a dissolution bath can have one temperature at any given time no matter which type of thermometer one could use to measure it. Similarly, just like the content of a tablet/capsule (amount of drug) can have one value at any given time, no matter which type of analytical technique one would use to measure it. Of course, different techniques/methods may provide different precisions due their nature, but results on average will be the same and technique independent.
It is not possible for the same tablet/capsule to have different weights at the same time. It is not possible that a dissolution bath can have different temperatures at the same time. It is not possible that a tablet/capsule can have different contents (amount of drug) at any given time.
If different balances, thermometers or analytical techniques provide different results, then this means that these are not measuring the values of the intended parameters but something else. Such techniques or testers are considered unreliable and, as a common practice, are not used any further.
On the other hand, in drug dissolution testing practice it is commonly accepted that each and every product can, and should, provide tester-dependent different dissolution value. A notable example in this regard is dissolution values for the USP prednisone PV Tablets, where paddle and basket provide two different drug dissolution values.
All four of the most commonly recommended dissolution testers are expected to provide different dissolution results for the same product. The analyst or formulator is free to choose, and rationalize, the one which suits his or her purpose, however, he or she will never be able to know the actual dissolution characteristics of a, or any, product. Presently, the drug release/dissolution characteristics are not product dependent, but tester dependent. Obviously, one cannot establish quality or bio-relevancy of any product as the results are not linked to the product but the tester.
It is such an unfortunate and bizarre practice and logic, not sure how this has been accepted and practiced for so long in the industry and regulatory environments. This is a serious anomaly which certainly requires urgent attention for correction.
Addressing the issue of failing calibration/PVT of dissolution testers (paddle/basket)
Problems of failures of calibration/ Performance Verification Testing (PVT) are not new. These have been with us since their introduction in the USP. Presently, the issue is not of the failures of calibration/PVT, which is very well established, but how it should be addressed.
To address the issue, let us breakdown the testing, calibration/PVT or drug dissolution testing in general, in different components.
(1) Calibration/PVT Tablets
(2) Dissolution testing procedure and/or analyst training/experience
(3) Apparatuses (paddle/basket)
(1) Calibration/PVT Tablets: At present there is no independent mechanism available or used to establish quality or reproducibility of the tablets. The quality and reproducibility of calibration/PVT are established using the paddle/basket apparatuses themselves. Therefore, quality and reproducibility of the tablets, hence failures, are dependent on the characteristics of the apparatuses used. Using statistical analyses, studies from the USP clearly demonstrated that PVT tablets do not significantly contribute to the failures (1, 2).
(2) Dissolution testing procedure and/or analyst training/experience: In this regard, one should note that dissolution testing means dropping a tablet/capsule into the vessel containing a medium maintained at 37ºC and starting the stirrer (spindle) at a pre-set rpm, followed by withdrawing a sample from the vessel. It is extremely important to note that an associated or required analytical technique such as spectrophotometry or chromatography is not part of the dissolution testing or testers. An analyst can conduct a dissolution test independent to the analytical (quantitation) technique and may send the samples to another analyst/analytical laboratory physically located hundreds of miles away to determine drug levels in the samples from dissolution testing using techniques of their choice. Continue reading
Developing a discriminatory test – nothing can be more confusing and wasting than this practice or requirement!
A discriminatory test reflects that it is capable of differentiating a bad batch/product from a good one. The question is how should one define bad vs good? In general a good product, in the context of dissolution testing, is a product that should be capable of releasing a drug from the product in the GI tract in an expected and reproducible manner. It is important to note that a drug release, or dissolution, test is linked to product behavior in the GI tract. On the other hand, it is a well-known fact that currently suggested dissolution methods/testers have no link to the product behavior in the GI tract (link). In fact, it is almost impossible to obtain reliable and physiologically relevant dissolution results using currently suggested apparatuses in particular paddle/basket apparatuses, because of the flaws of the testers (link). Therefore, by extension, it is almost impossible to have discriminatory dissolution methods to differentiate a bad product from a good product.
The irrelevancy of current practices of developing discriminatory tests, methods or testers may also be explained in another way with the following analogy. For example, one may ask if anyone has seen a discriminatory thermometer, laboratory balance, pH meter, spectrophotometer, chromatograph etc. The answer is, not really; because all one does, by using a tester/method, is to measure the value of the parameter. The test/method is used only to determine value/result and the scientist/analyst uses this value for interpretation of the characteristics of the product. For example, one never says, requires or develops a discriminatory thermometer, when one monitors the temperature. One uses the thermometer to measure the value (temperature) of the corresponding parameter (body temperature). The thermometer never tells if a person has a fever or not. It only tells the temperature, but a physician interprets it as a fever or any other deviations. So, why does one have discriminatory dissolution methods or testers? It is simply to market the made-up science and flawed testers. Developing of a discriminatory test/method, as practiced or required, has no meaning and serves no useful purpose. One requires a dissolution tester or method to measure dissolution characteristics of a product reflecting its in vivo dissolution characteristics. Once one has such a method, it automatically becomes a discriminatory test. One does not require any extra effort or steps to make it a discriminatory test. The dissolution results thus obtained using such a method/tester would reflect dissolution characteristics of a product and then the analyst/formulator is to decide whether the product is of acceptable quality/characteristics or not. Continue reading